A kind of halogenated oxalylamine compound and its preparation method and application
A technology of halogenated oxalylamine and heteroallylamine, which is applied in the field of halogenated oxalylamine compounds and their preparation, can solve the problem of no application prospect, limited substrate applicability, Problems such as halogenated oxalylamine cannot be applied to achieve the effect of novel and efficient synthesis method, wide substrate applicability, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
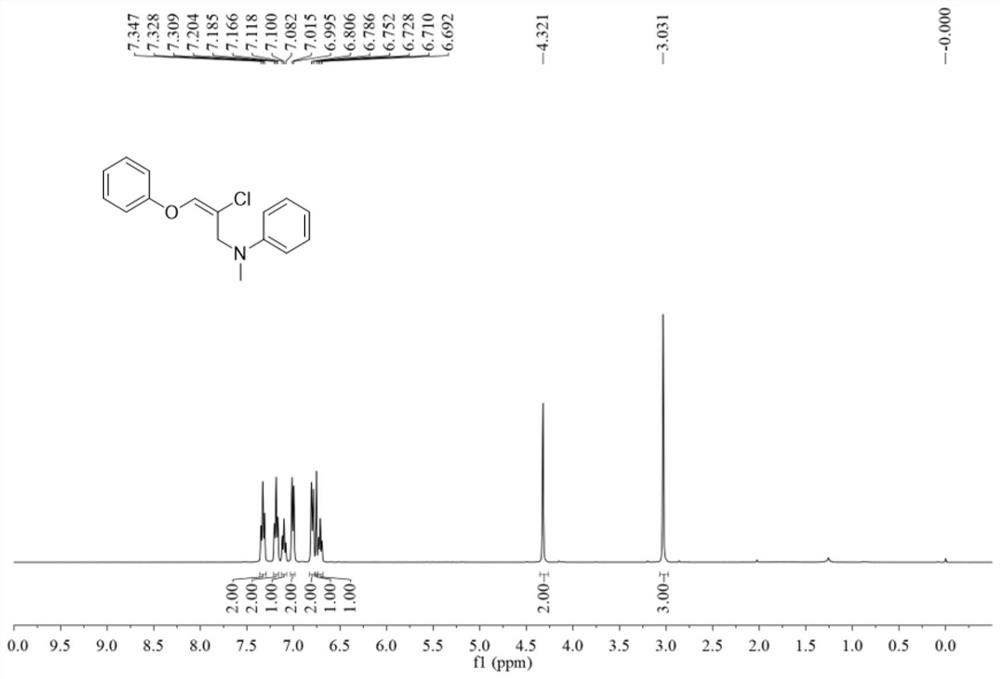
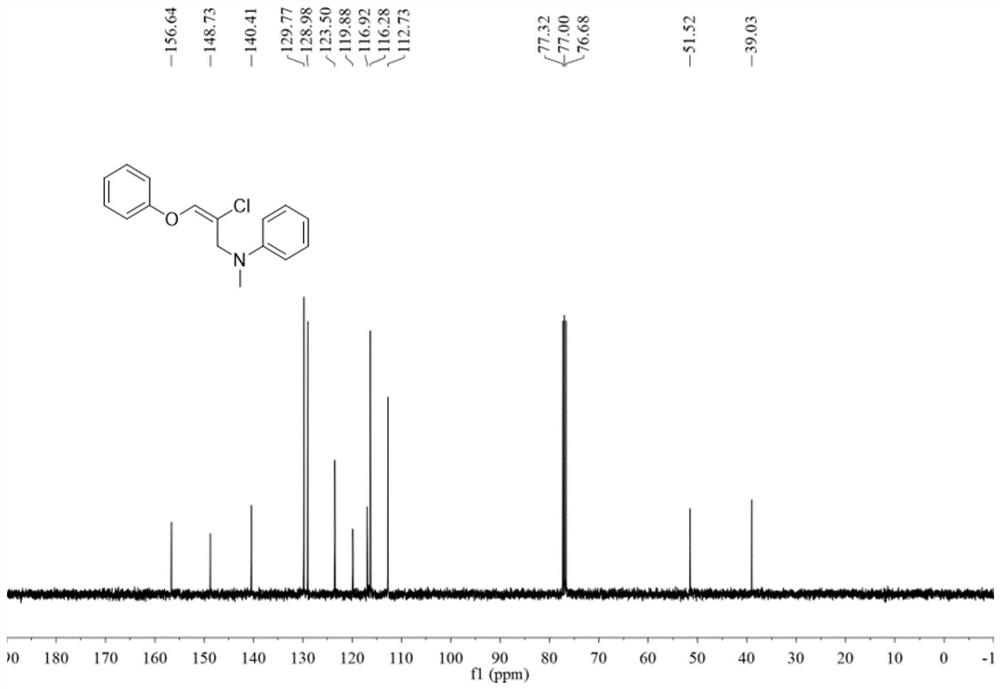
[0063] In the test tube, first add 0.12 mmol of copper chloride in sequence, dissolve it in 1.0 ml of 1,4-dioxane solvent, and finally add 0.2 mmol of 2-methyl-N-methylaniline and 0.3 mmol of phenyl Allene ether was covered with an oxygen balloon, stirred and reacted at 40 degrees Celsius at 700 rpm for 6 hours, and then stopped stirring. Add 5 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous sodium sulfate to dry, filter, concentrate under reduced pressure, and then separate and purify by thin-layer chromatography to obtain the target product, the thin-layer chromatography used The developer is petroleum ether:ethyl acetate mixed solvent with a volume ratio of 500:1, and the yield is 82%.
[0064] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows image 3 and Figure 4 shown; the structural characterization data are shown below:
[0065] 1 H NMR (...
Embodiment 3
[0072] In the test tube, first add 0.12 mmol of copper chloride in sequence, dissolve it in 1.0 ml of 1,4-dioxane solvent, and finally add 0.2 mmol of 2-bromo-N-methylaniline and 0.3 mmol of phenyl bismuth Diene ether was covered with an oxygen balloon, stirred and reacted at 40 degrees Celsius at 700 rpm for 6 hours, and then stopped stirring. Add 5 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous sodium sulfate to dry, filter, concentrate under reduced pressure, and then separate and purify by thin-layer chromatography to obtain the target product, the thin-layer chromatography used The developer is petroleum ether:ethyl acetate mixed solvent with a volume ratio of 500:1, and the yield is 68%.
[0073] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows Figure 5 and Figure 6 shown; the structural characterization data are shown below:
[0074] 1 ...
Embodiment 4
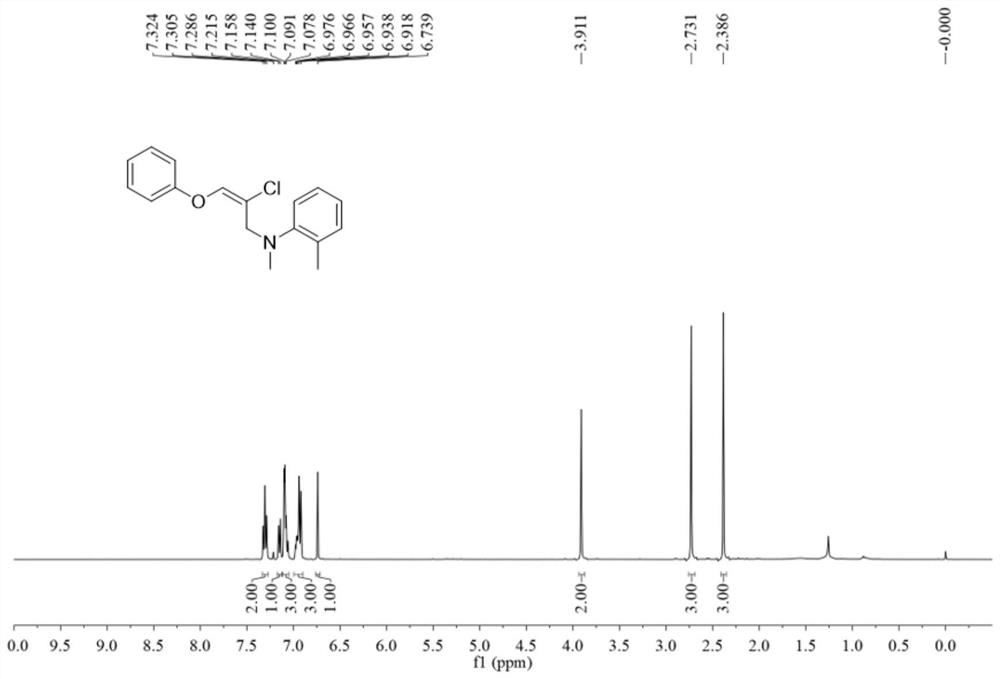
[0081] In the test tube, first add 0.12 mmol of copper chloride in sequence, dissolve it in 1.0 ml of 1,4-dioxane solvent, and finally add 0.2 mmol of 4-methyl-N-methylaniline and 0.3 mmol of phenyl Allene ether was covered with an oxygen balloon, stirred and reacted at 40 degrees Celsius at 700 rpm for 6 hours, and then stopped stirring. Add 5 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous sodium sulfate to dry, filter, concentrate under reduced pressure, and then separate and purify by thin-layer chromatography to obtain the target product, the thin-layer chromatography used The developer is petroleum ether:ethyl acetate mixed solvent with a volume ratio of 500:1, and the yield is 68%.
[0082] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows Figure 7 and Figure 8 shown; the structural characterization data are shown below:
[0083] 1 H NMR ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com