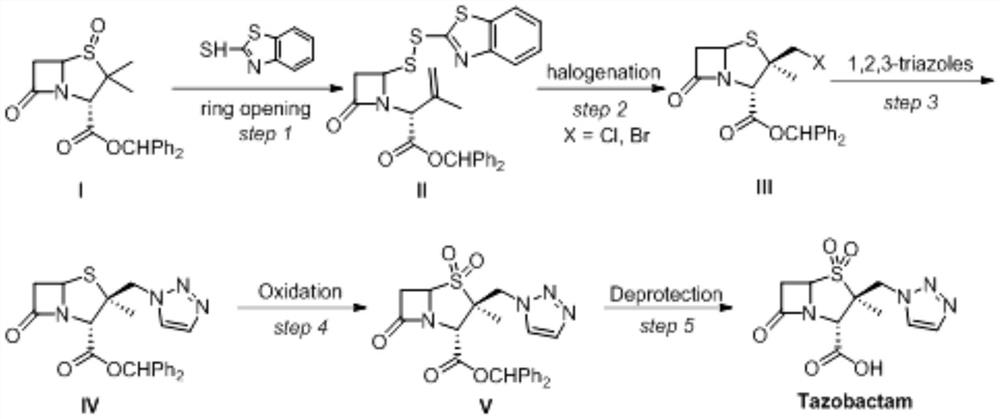
Method for electrochemical synthesis of tazobactam key intermediate
A tazobactam, electrochemical technology, applied in the field of electrochemical synthesis of key intermediates of tazobactam, can solve the problems of low reaction efficiency, low selectivity, poor economy and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
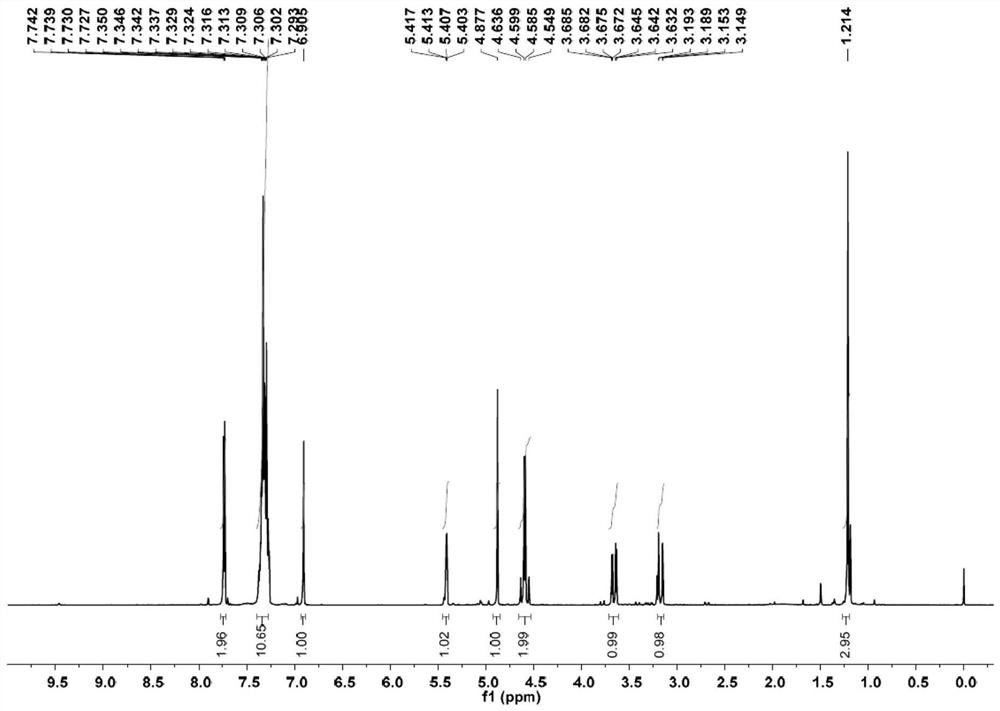
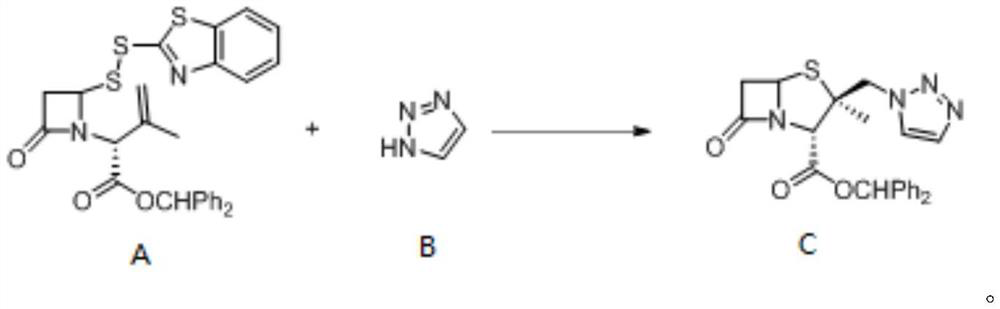
[0039] Example 1: Preparation 2β-triazole methylmirinate C
[0040]
[0041] At room temperature, a magneton was added to the non-separated electrolysis tank (8 mL), and a double sulfur-randoma A (133 mg, 0.25 mmol), 1,2,3-triazole B (138 mg, 2 mmol,) were added to the slot. 8EQ), tetrabutyate (185 mg, 0.5 mmol, 2 eq). Acetonitrile (4.5 mL) and acetic acid (0.5 ml) were added using a syringe. After all materials are added, the graphite sheet (1 × 7 × 0.3cm) is equipped. 3 ) And platinum tablet (1 × 7 × 0.3cm 3 ), Electrode immersion area is 2cm 2 (At this time, the current density is 4mA / cm 2 ). The reaction mixture was stirred for 10 min, and the DC power supply was adjusted to constant current mode, and 8 mA electrolysis 5f was completely converted. After the end of the reaction, the reaction liquid sampled from the HPLC external standard, the yield was 76.8%, and the column chromatography was separated to obtain a white solid, and the yield was 76.2%.
[0042] Nuclear magne...
Embodiment 2
[0043] Example 2: Carbon felt as an anode prepared 2β-triazole methylmidin-dibenzyl
[0044] At room temperature, a magneton was added to the non-separated electrolysis tank (8 mL), and a double sulfur-randoma A (133 mg, 0.25 mmol), 1,2,3-triazole B (138 mg, 2 mmol,) were added to the slot. 8EQ), tetrabutyate (185 mg, 0.5 mmol, 2 eq). Acetonitrile (4.5 mL) and acetic acid (0.5 ml) were added using a syringe. After all materials are added, equipment is equipped with carbon felt (1 × 7cm 2 ) And platinum tablet (1 × 7 × 0.3cm 3 As the yin and inferior, the electrode immersion area is 2 cm 2 . The reaction mixture was stirred for 10 min, and the DC power supply was adjusted to constant current mode, and 8 mA electrolysis 5f was completely converted. After the reaction, the reaction liquid was sampled from the HPLC external standard, and the yield was 71.0%.
Embodiment 3
[0045] Example 3: Tablet as a cathode Preparation 2β-triazol methylpatoenenenate C
[0046] At room temperature, a magneton was added to the non-separated electrolysis tank (8 mL), and a double sulfur-randoma A (133 mg, 0.25 mmol), 1,2,3-triazole B (138 mg, 2 mmol,) were added to the slot. 8EQ), tetrabutyate (185 mg, 0.5 mmol, 2 eq). Acetonitrile (4.5 mL) and acetic acid (0.5 ml) were added using a syringe. After all materials are added, the graphite sheet (1 × 7cm) is equipped. 2 ) And iron sheet (1 × 7 × 0.2cm 3 As the yin and inferior, the electrode immersion area is 2 cm 2 . The reaction mixture was stirred for 10 min, and the DC power supply was adjusted to constant current mode, and 8 mA electrolysis 5f was completely converted. After the reaction, the reaction liquid sampled the HPLC external standard, yield 49.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com