High-selectivity catalytic synthesis method for 4, 4'-bisphenol F
A high-selectivity, catalyst technology, used in chemical instruments and methods, preparation of organic compounds, catalysts for physical/chemical processes, etc. To achieve the effect of improving acidity and steric hindrance, good stability and good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
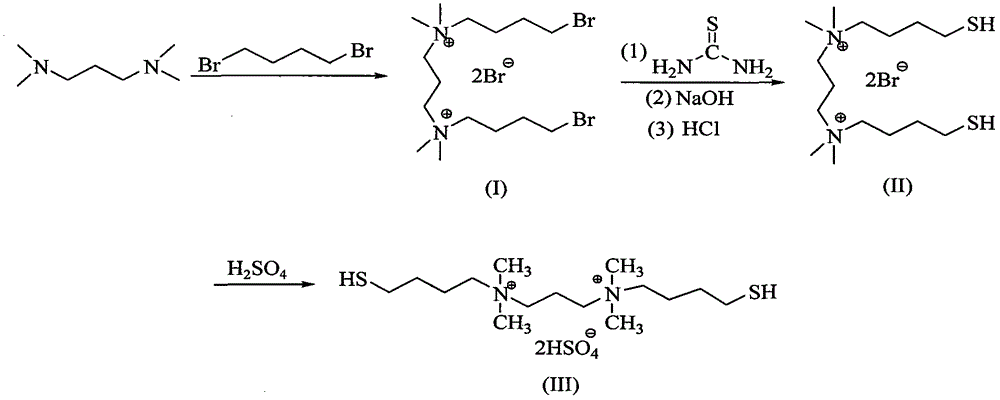
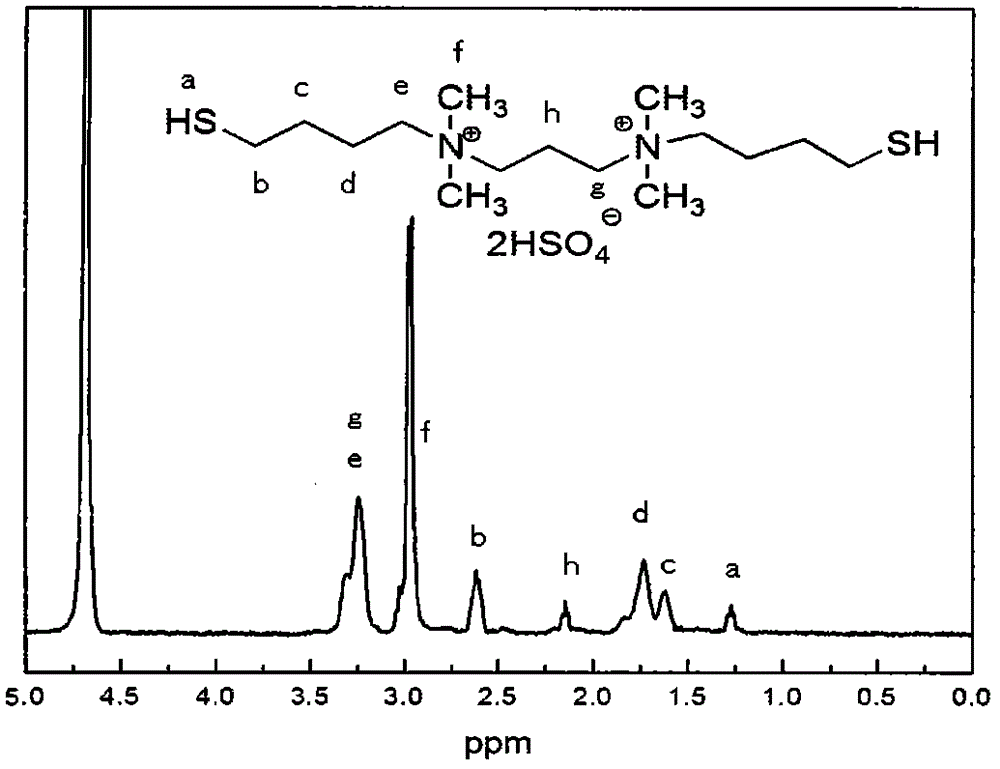
[0019] (1) Preparation of ionic liquid catalyst N,N-bis(4-mercapto)butyl-N,N,N,N-tetramethyl-1,3-propanediammonium dihydrogensulfate
[0020] The first step: 86.4 grams of 1,4 dibromobutane and 40 grams of tetrahydrofuran are mixed and dissolved to form solution A, and 13 grams of N,N,N,N-tetramethyl-1,3-propanediamine and 50 grams of methanol Mix and dissolve to form solution B. Slowly add solution B to solution A under stirring, react at 0°C for 3 hours, then raise the temperature to 25°C and continue to react for 24 hours. After the reaction, tetrahydrofuran and methanol are evaporated by fractional distillation, and extracted with water , the water phase is evaporated to obtain the first step reaction product, and the organic phase is excess 1,4-dibromobutane, which is directly recycled and used;
[0021] The second step: mix and dissolve 15.3 grams of thiourea and 75 grams of ethanol to form solution C, mix and dissolve 51 grams of the reaction product of the first step a...
Embodiment 2
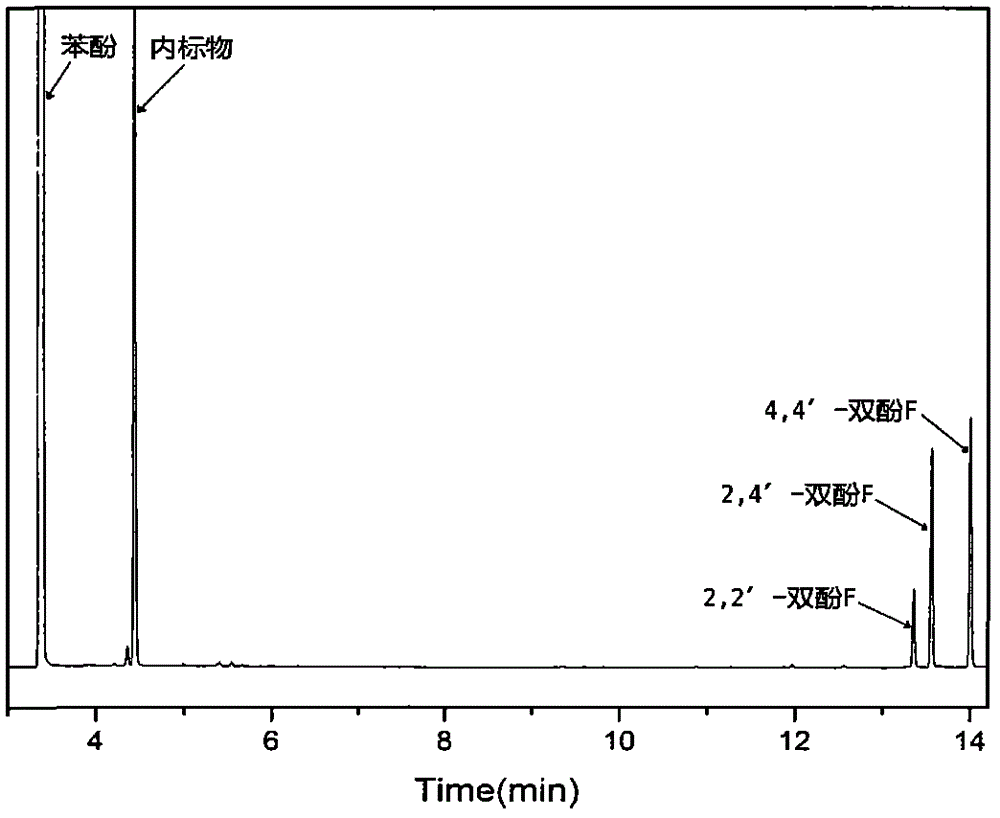
[0030] Similar to Example 1, the difference is only that the mol ratio of phenol to ionic liquid to formaldehyde is 10:1:1 in the process of synthesizing bisphenol F, and all the other processes are the same, and the yield of bisphenol F obtained is 80%, 4 , 4'-bisphenol F selectivity was 62%.
Embodiment 3
[0032] Similar to Example 1, the difference is only that the molar ratio of phenol to ionic liquid to formaldehyde is 4:1:1 in the process of synthesizing bisphenol F, and all the other processes are the same, and the yield of bisphenol F obtained is 51%, 4, 4'-Bisphenol F selectivity was 58%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com