Synthesis process of tetrabutylammonium bromide
A technology of tetrabutylammonium bromide and synthesis process, applied in the field of synthesis technology of tetrabutylammonium bromide, can solve problems such as long reaction time, complicated post-processing operation, etc., and achieve short reaction time, good yield and purity , the effect of simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Preparation of photocatalyst TiO 2 / NiO
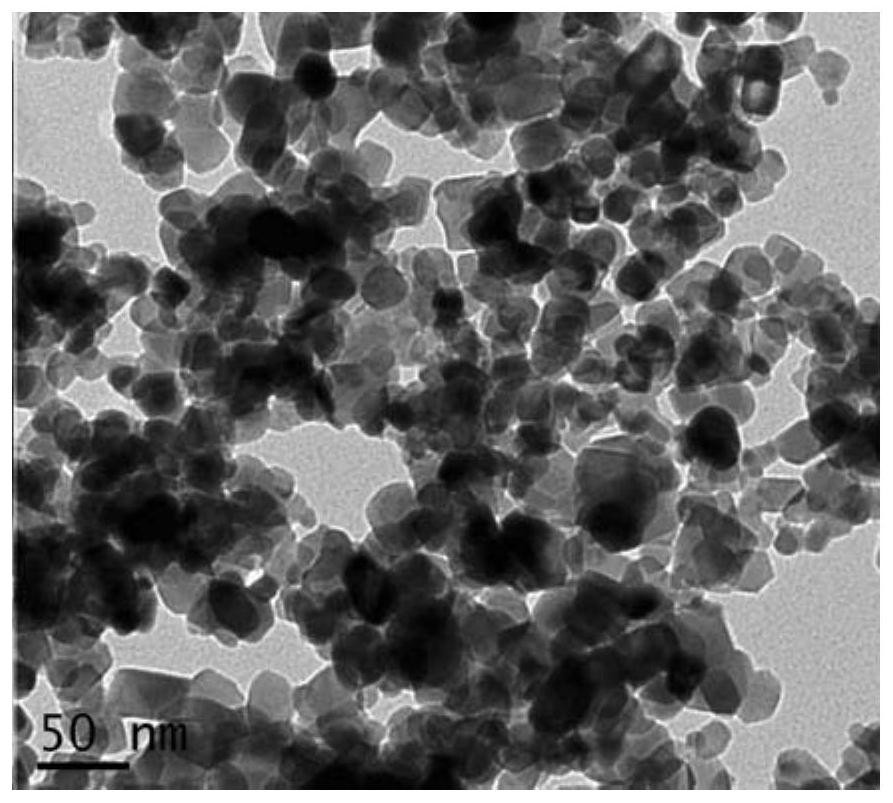
[0043] 1g of TiO 2 (P25) powder is added to the solution of nickel acetylacetonate (the solution concentration is kept at 6.0×10 - 4 mol / L~7.0×10 -4 mol / L or so), it was placed in a three-necked flask, and mechanically stirred for 24 hours at room temperature. The final product was washed repeatedly with the same solvent to remove unreacted complexes, and dried at room temperature. Then bake in a muffle furnace at 400°C to 500°C for 1h, repeat the above steps to obtain the photocatalyst TiO 2 / NiO (10.5%).
[0044] The synthesized photocatalyst TiO of embodiment 1 of the present invention 2 / NiO(10.5%) scanning electron microscope picture as figure 1 shown.
Embodiment 2
[0045] Embodiment 2: Preparation of photocatalyst TiO 2 / Fe 2 o 3
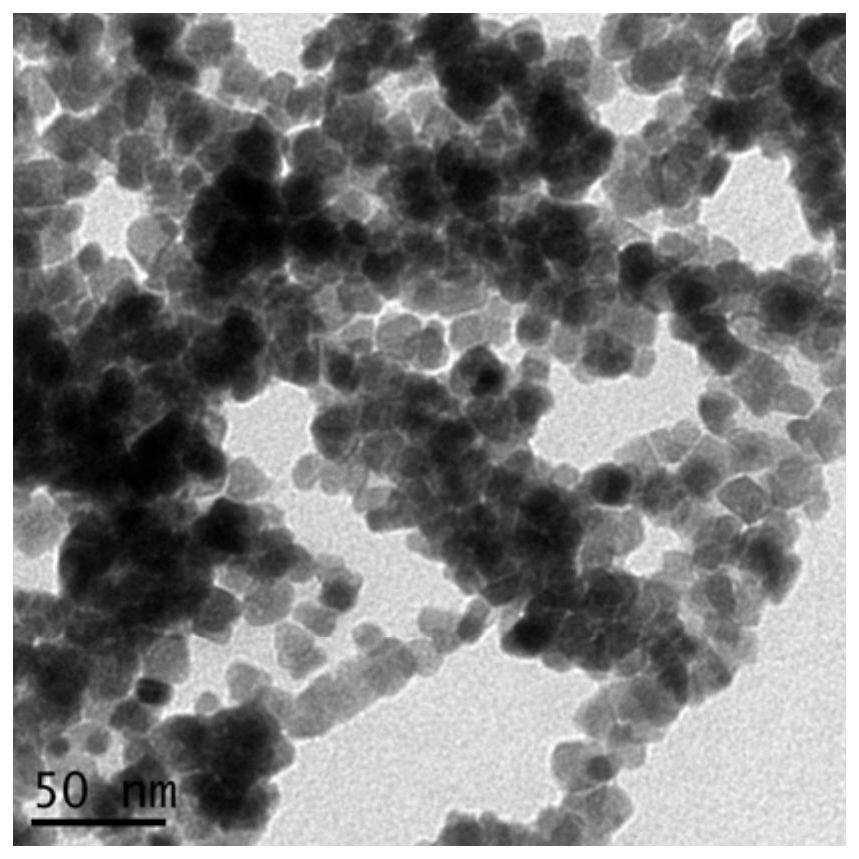
[0046] Iron acetylacetonate was added to the mixed solvent of 15ml ethanol and 85ml n-hexane, and the solution concentration was kept at 6.5×10 -4 mol / L, then add 1g of TiO 2 (P25), placed in a three-necked flask, mechanically stirred at room temperature for 24 hours, washed repeatedly with the same solvent, dried at room temperature, and calcined at 400°C to 500°C for 1 hour. Repeat the above steps to obtain different photocatalyst TiO 2 / Fe 2 o 3 (6.5%).
[0047] The synthesized photocatalyst TiO of embodiment 2 of the present invention 2 / Fe 2 o 3 (6.5%) scanning electron microscope picture as figure 2 shown.
Embodiment 3
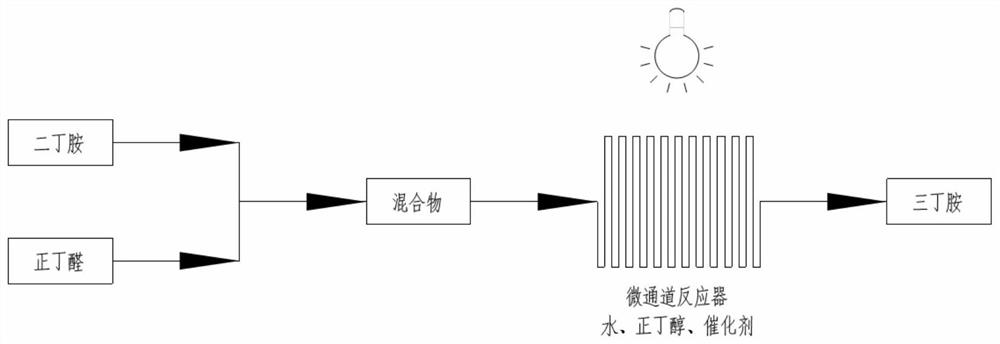
[0048] Embodiment 3: synthetic tributylamine
[0049] With 50g dibutylamine (0.387mol), 29.29g butyraldehyde (0.406mol), photocatalyst TiO 2 / NiO (10.5%) 1g, n-butanol 100mL, deionized water 10mL were added to the mixer and mixed evenly, pumped into the microchannel reactor, the flow rate was controlled to 10mL / min, under the 100W LED white light After reacting for 2-4 hours under irradiation, monitor the reaction to the end point, filter to remove the photocatalyst, concentrate to remove the solvent, and wash the catalyst once with ethanol and n-butanol, respectively, and then use it for the second catalytic reaction. Gas phase monitoring reaction yield was 90%, purity: 97%.
[0050] Alternate example:
[0051] The preparation method is the same as in Example 3, the difference is that the type and amount of the catalyst and the flow rate of the microchannel reactor are adjusted, and its influence on the reaction is tested respectively, as shown in Table 1.
[0052] Table 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com