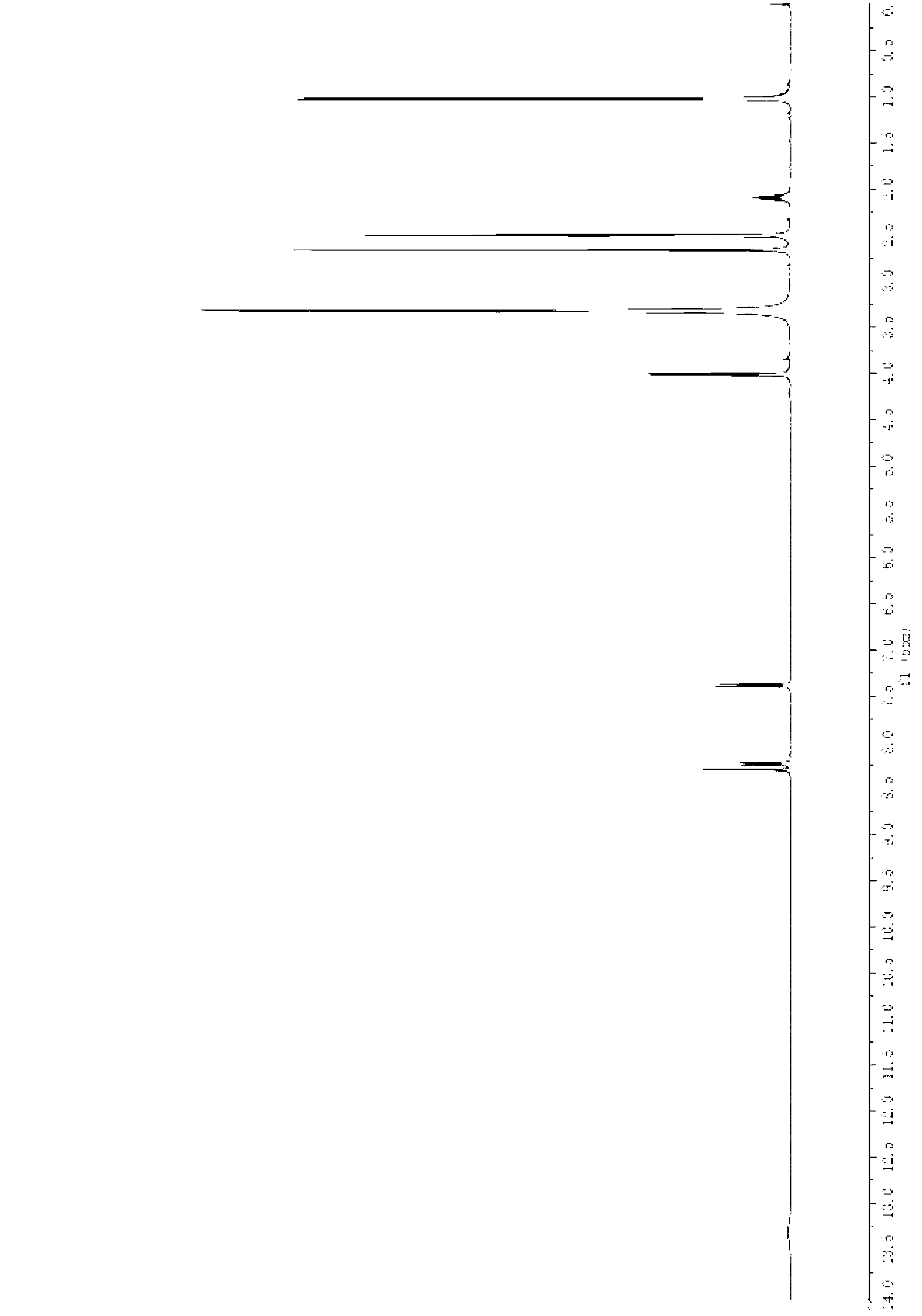
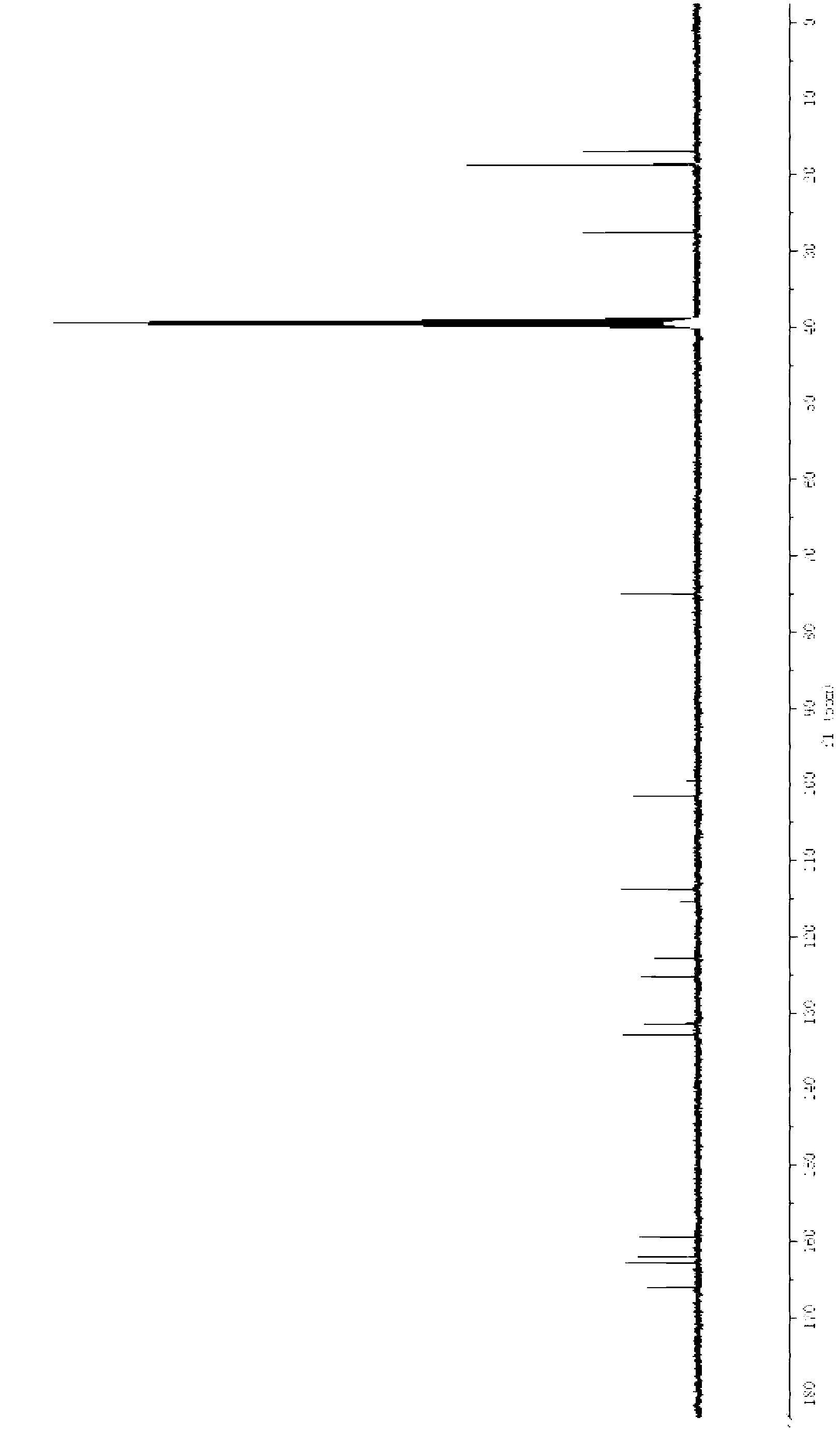
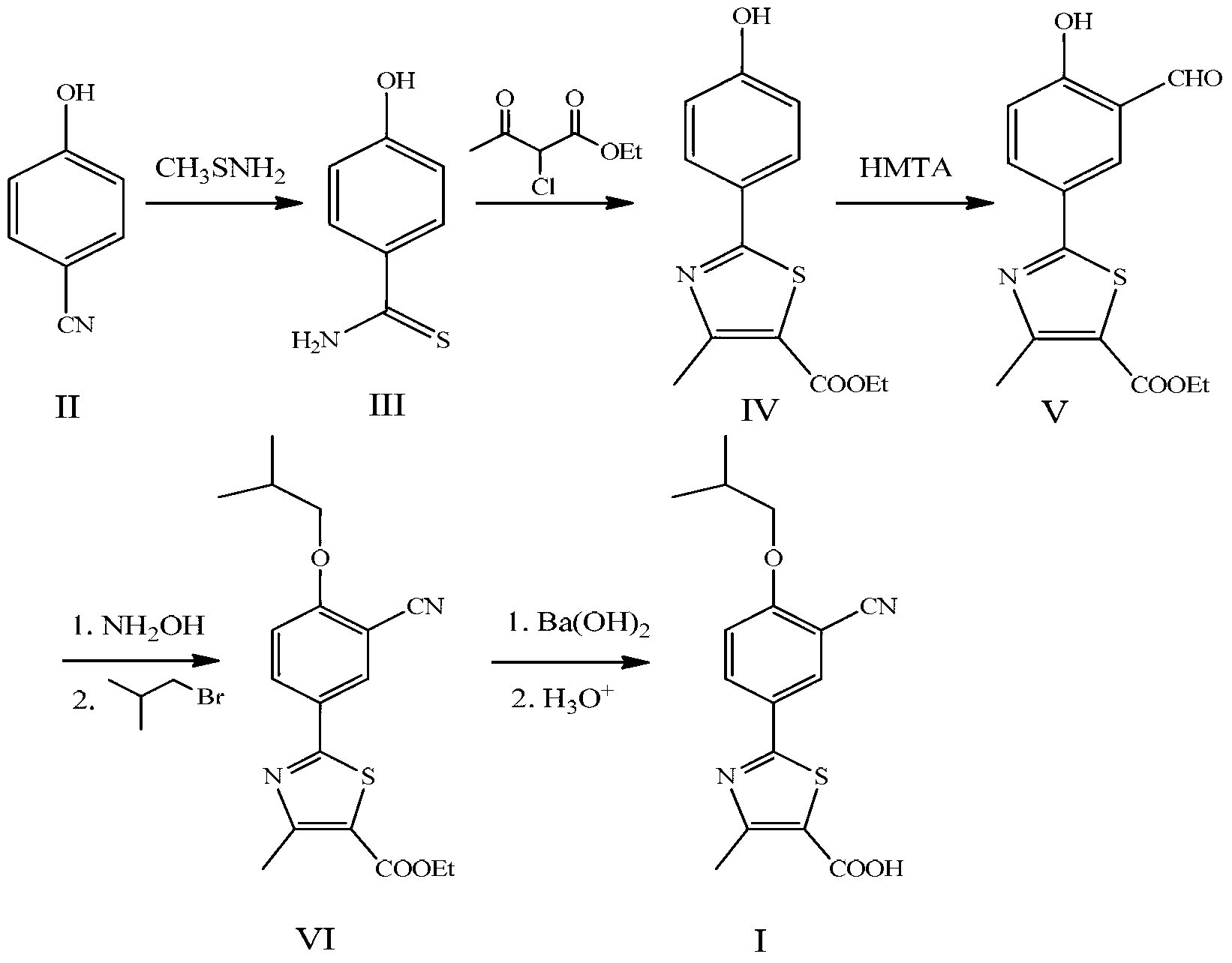
Preparation method for febuxostat
A febuxostat and reaction technology, applied in the field of preparation of febuxostat, can solve the problems of increasing the difficulty of post-processing operations, low total yield of febuxostat, unfavorable environment, etc. Conducive to industrial scale-up production and the effect of safe reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A preparation method of febuxostat, the specific steps are as follows:
[0044] (1) Add 200mL of concentrated hydrochloric acid (concentration: 12mol / L) into the flask, add 13.0g of thioacetamide under mechanical stirring, add 10.0g of 4-hydroxybenzonitrile after complete dissolution, stir at 50°C for 4 hours, cool down, A large amount of solids precipitated, filtered, washed with a small amount of water until neutral, and dried to obtain 12.3 g of intermediate (III), with a yield of 95.8%.
[0045] (2) Dissolve 12.3g of intermediate III in 60mL of absolute ethanol, heat to reflux, slowly add 14.2g of ethyl 2-chloroacetoacetate dropwise, and continue to reflux for 5 hours. After the reaction is complete, add it to an ice-water bath to cool. A large amount of solids were precipitated, filtered and dried to obtain 20.4 g of intermediate IV as a yellow solid, with a yield of 96.0%.
[0046] (3) Dissolve 20.4g of intermediate IV in a mixture of 90mL of methanesulfonic acid a...
Embodiment 2
[0050] The difference between this embodiment and embodiment 1 is:
[0051] Add 200mL of dilute hydrochloric acid (concentration: 3mol / L) into the flask, add 13.0g of thioacetamide under mechanical stirring, add 10.0g of 4-hydroxybenzonitrile after complete dissolution, stir at 50°C for 4 hours, cool, and there are a lot of solids Precipitate, filter, wash with a small amount of water until neutral, and dry to obtain 9.8g. The yield of 4-hydroxythiobenzamide is 76.6%.
Embodiment 3
[0053] The difference between this embodiment and embodiment 1 is:
[0054] Dissolve 20.4g of intermediate IV in 110mL of methanesulfonic acid and 10mL of trifluoroacetic acid, heat to 90°C, add 27.9g of hexamethylenetetramine three times under mechanical stirring (half an hour apart), and continue the reaction for 8 hours. Add 300mL ice-cold salt water, stir for 1 hour, a large amount of yellow solid precipitates, filter, wash with water until neutral, dry to obtain 16.7g, 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5 - The yield of ethyl carboxylate is 74.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com