Febuxostat crystal and preparation method and application in medicines thereof
A febuxostat and crystal technology, applied in the field of febuxostat crystals, preparation and application in medicine, can solve the problems of unsatisfactory dissolution results and needs to be improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 10g of the crude febuxostat and 30ml of N,N-dimethylformamide (DMF) into a 250ml round-bottomed flask, stir and raise the temperature to 100°C to make the solids completely dissolved, and then lower the temperature to 20°C. Add 60ml of water while stirring. Incubate and crystallize for 2 hours, filter, wash the filter cake with water, and vacuum dry at 80°C for 10 hours to obtain 7.9 g of light yellow crystalline powder with a purity of ≥99% (HPLC.
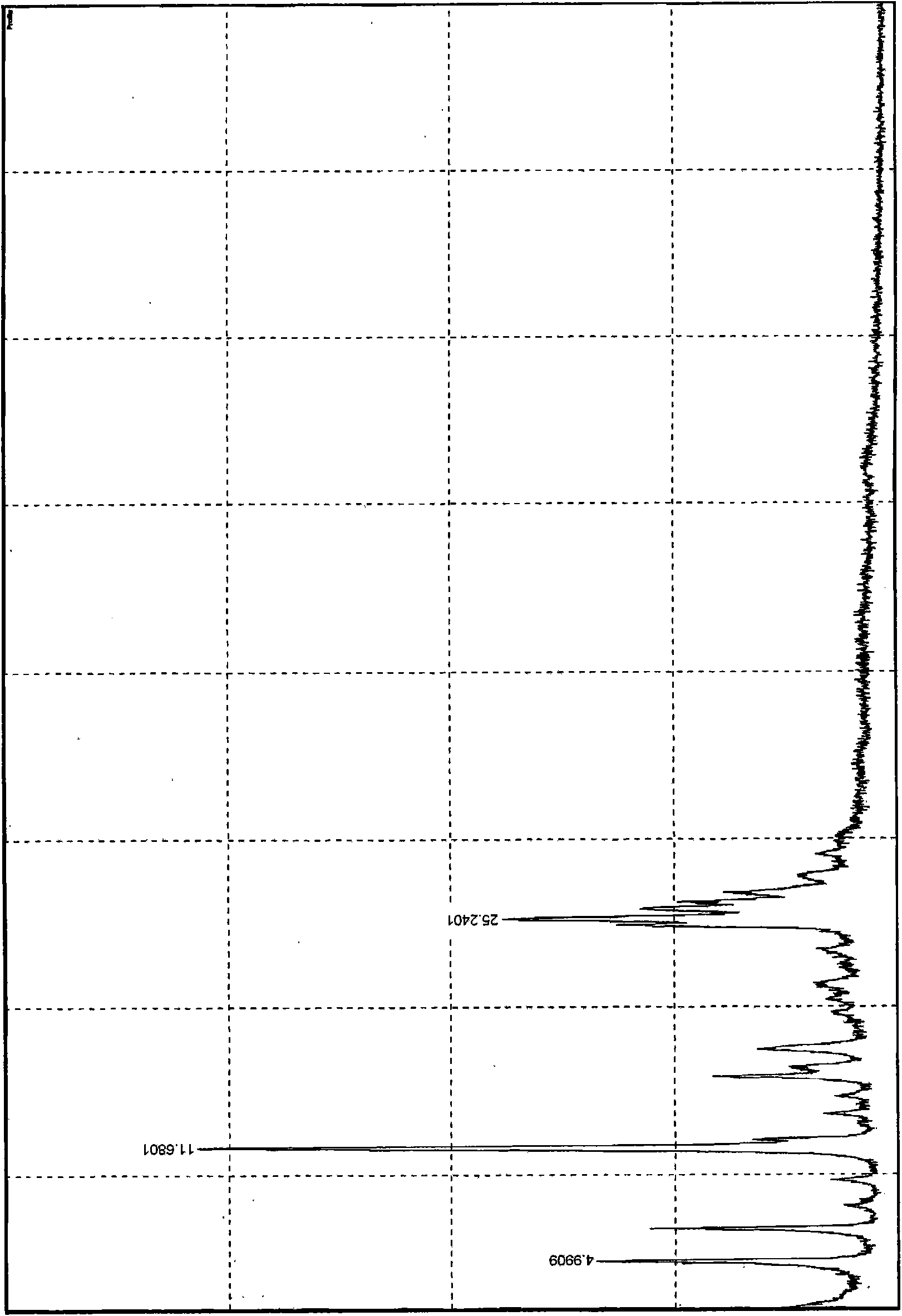
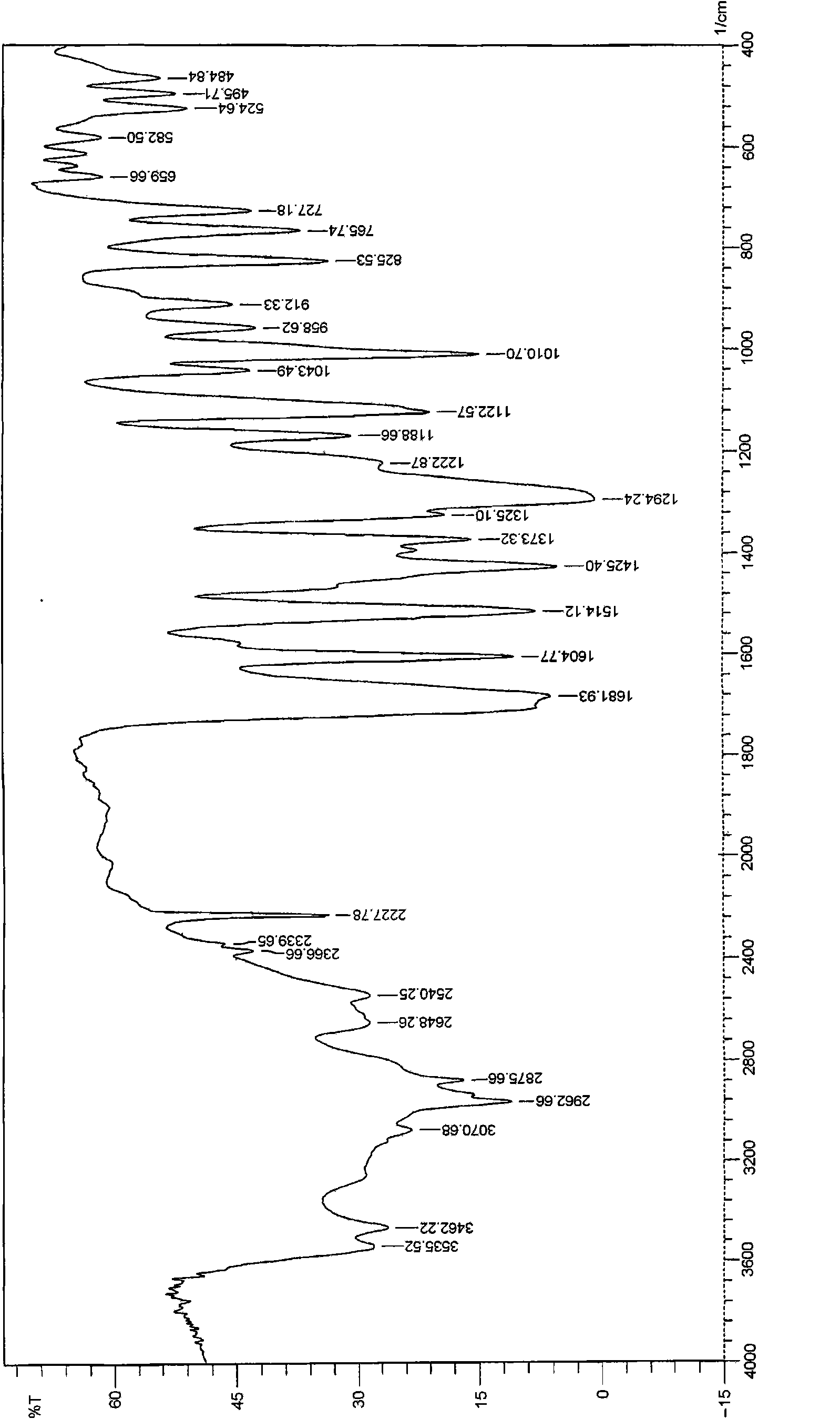
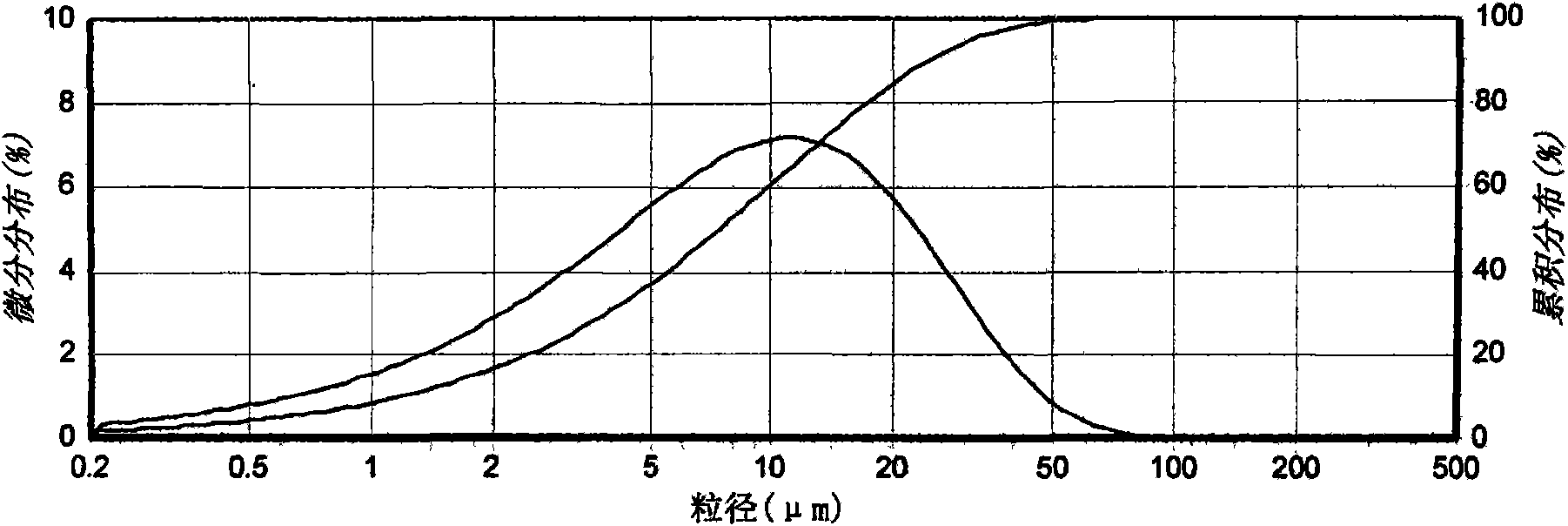
[0020] Take a sample to determine the reflection angle 2θ absorption peak of its X-ray powder diffraction as figure 1 As shown, the absorption peak data are shown in Table 1; the infrared spectrogram and the crystal particle size distribution curve are shown in Figure 2 ~ Figure 3 Shown. by figure 1 As can be seen from Table 1, the reflection angle 2θ of the X-ray powder diffraction pattern of the crystal is about 4.99°, 6.90°, 11.68°, 15.89°, 17.54°, 24.87°, 25.24°, 25.82°, 26.18° and 26.76° There are characteristic abs...
Embodiment 2
[0024] Add 10g of the crude febuxostat and 100ml of N,N-dimethylformamide (DMF) into a 1000ml round bottom flask, stir and raise the temperature to 60~80℃, so that the solids are fully dissolved, keep at 55℃~60℃, and stir. 600ml of water was added dropwise to the bottom, the temperature was kept for 1 hour to crystallize, filtered, the filter cake was washed with water, and dried under vacuum at 80°C for 15 hours to obtain 8.2 g of light yellow crystalline powder with a purity of ≥99% (HPLC normalization method). Sampling and testing showed the same N crystal form; particle size analysis showed the same crystallite state.
[0025] Table 1 Reflection angle 2θ absorption peak data of crystal X-ray powder diffraction
[0026]
[0027] Table 2 Test results of particle size distribution
[0028]
Embodiment 3
[0030] Add 10g of the crude febuxostat and 50ml of N,N-dimethylacetamide to a 500ml round-bottomed flask in turn, stir and raise the temperature to 80-100°C to dissolve the solids, and cool down to -5°C to 0°C in an ice bath. 500ml of water was added dropwise with stirring, the temperature was kept for 1 hour to crystallize, filtered, the filter cake was washed with water, and vacuum dried at 80°C for 12 hours to obtain 9.0 g of light yellow crystalline powder with a purity of ≥99% (HPLC normalization method). Sampling and testing showed that the formation was crystal form N; particle size analysis showed that it was microcrystalline.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com