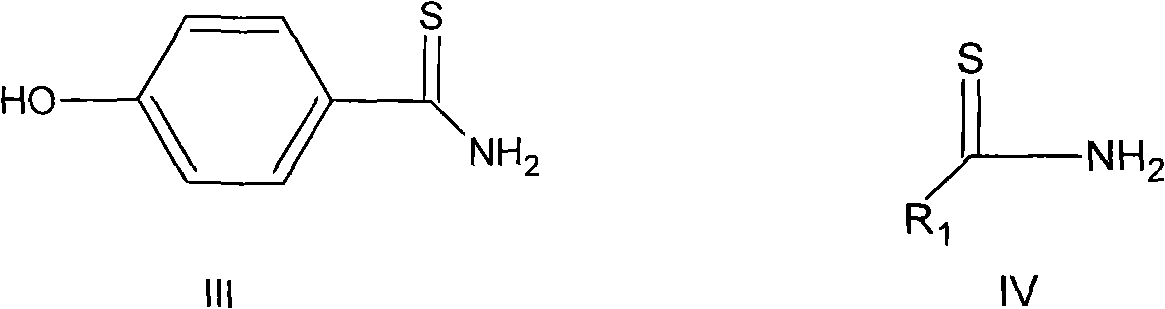
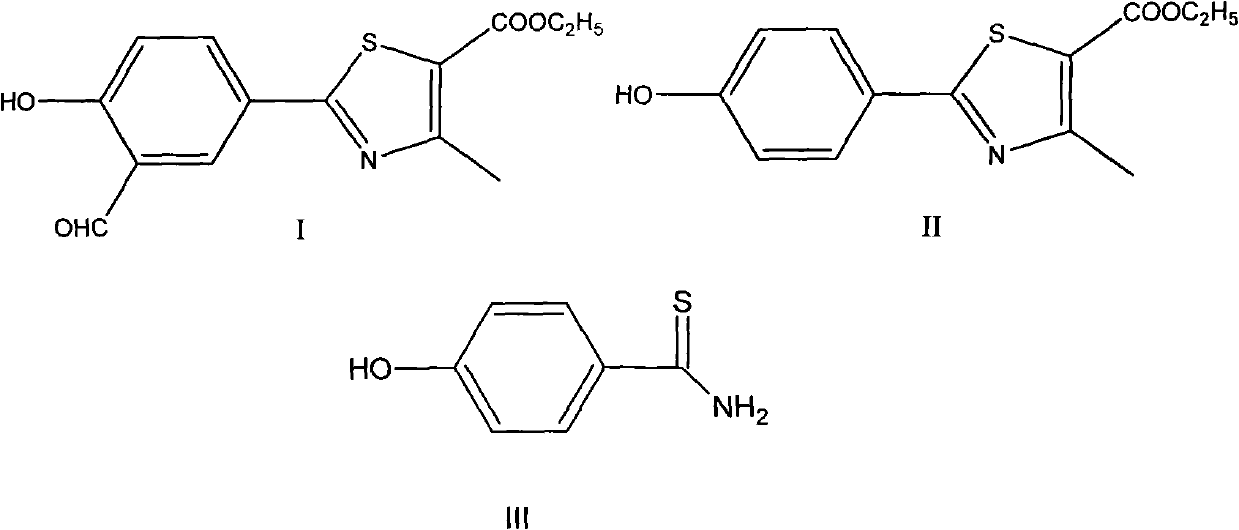
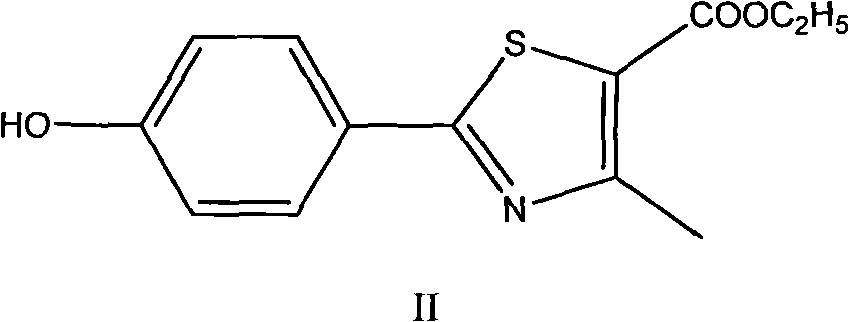
Preparation method of intermediates of Febuxostat
A technology of reaction time and compound, applied in the direction of organic chemistry, etc., can solve the problems of cumbersome post-processing, cumbersome post-processing, low yield, etc., and achieve the effects of eliminating high-temperature and high-pressure reactions, cheap and easily available raw materials, and simple reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] In a 3L reaction flask, add 1200ml of concentrated hydrochloric acid (concentration: 12mol / L), under stirring, add 92g of thioacetamide, wait until it is completely dissolved, then add 96g of p-cyanophenol, and react with stirring at 40°C for 0.5 hours, A large amount of light yellow solid precipitated, and the reaction was continued for 3 hours, the reaction was stopped, cooled, a large amount of solid precipitated, filtered, the filter cake was rinsed with a small amount of water, and 112.2 g of the product was obtained after drying. Yield: 91.0%.
Embodiment 2
[0051] In a 0.5L reaction flask, add 300ml of dilute hydrochloric acid (concentration: 6mol / L), under stirring, add 28g of thioacetamide, wait until it is completely dissolved, then add 24g of p-cyanophenol, and stir for 3 hours at 50°C. After stopping the reaction, cooling, a large amount of solids precipitated out, suction filtered, and the filter cake was washed with a small amount of water to obtain 41.8 g of the product with a water content of 35.1%. Yield: 88.3%.
Embodiment 3
[0053] In a 100ml reaction bottle, add 40ml of 50% concentrated sulfuric acid, under stirring, add 8.9g of thioacetamide, after the dissolution is complete, then add 9.6g of p-cyanophenol, stir and react at 60°C for 5 hours, stop the reaction, and cool , a large amount of solids were precipitated, filtered, the filter cake was rinsed with a small amount of water, filtered with suction, and dried to obtain 9.7 g of the product. HPLC: 98.9%, yield: 78.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com