Solid dispersion system of febuxostat and preparation method of solid dispersion system, and pharmaceutical applications
A technology of solid dispersion and febuxostat, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., which can solve the limitations of absorption and utilization, active ingredients are not easy to wet and diffuse, and biological Problems such as low utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
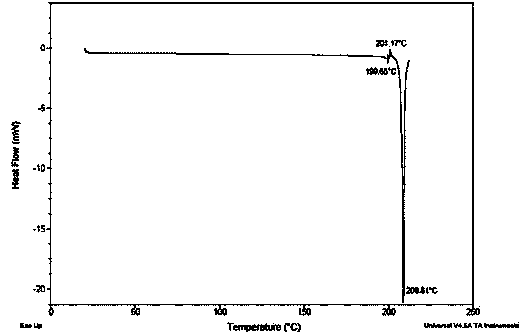
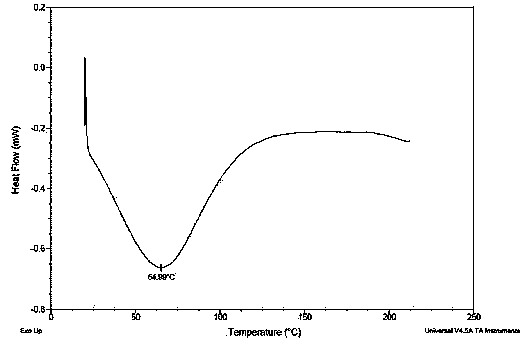
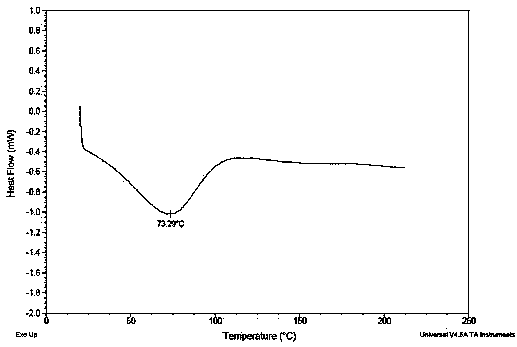
Image
Examples
Embodiment 1
[0036] prescription:
[0037] Febuxostat: 100mg
[0038] Colloidal silicon dioxide: 600mg
[0039] Dissolve 100mg of febuxostat in an appropriate amount of absolute ethanol, add 600mg of colloidal silica under magnetic stirring, sonicate for 30min, fully stir at room temperature for 6h, and then spin-evaporate under reduced pressure at 40℃ to release the dry solvent , Collect the solid, grind and smash to obtain the febuxostat solid dispersion system.
Embodiment 2
[0041] prescription:
[0042] Febuxostat: 100mg
[0043] Colloidal silicon dioxide: 300mg
[0044] PVP: 300mg
[0045] Dissolve 100mg of febuxostat in an appropriate amount of absolute ethanol or dichloromethane. Under magnetic stirring, add 300mg of colloidal silicon dioxide to ultrasonic for 30min, and add 300mg of PVP to the above solution, and mix with the drug and the drug under stirring. The carrier materials are mixed; fully stirred at room temperature for 6 hours, and then rotated and evaporated under reduced pressure at 40° C. to release the dry solvent, collect the solid, grind and pulverize, to obtain the febuxostat solid dispersion system.
[0046]
Embodiment 3
[0048] prescription:
[0049] Febuxostat: 100mg
[0050] Colloidal silicon dioxide: 300mg
[0051] HPMC: 300mg
[0052] Dissolve 100 mg of febuxostat in an appropriate amount of dichloromethane, add 300 mg of colloidal silica under magnetic stirring for 30 minutes, and add 300 mg of HPMC to the above solution, and mix with the drug and carrier material under stirring; Stir fully at room temperature for 6 hours, and then rotate and evaporate under reduced pressure at 40° C. to release the dry solvent, collect the solid, grind and pulverize, to obtain the febuxostat solid dispersion system.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com