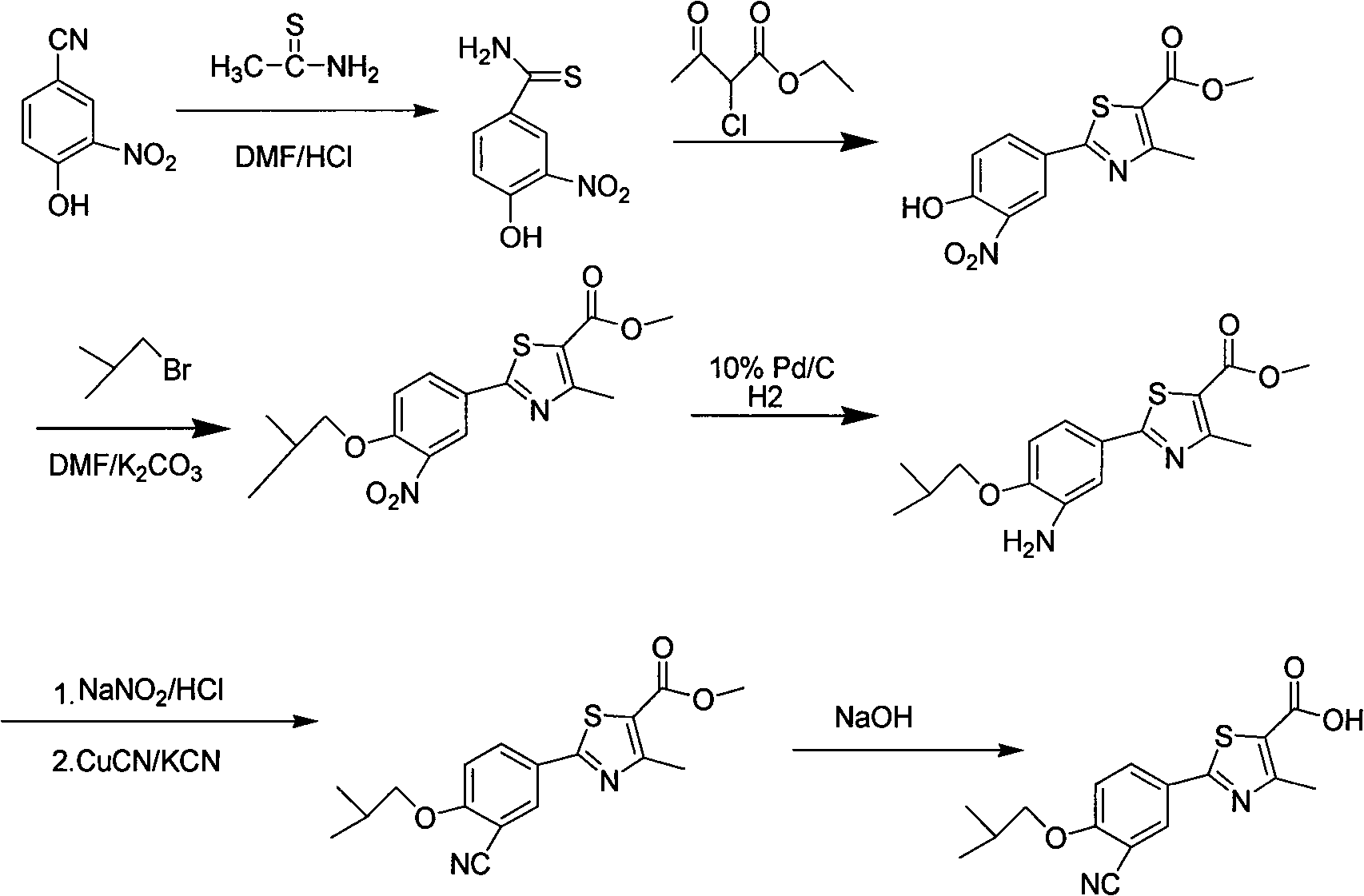
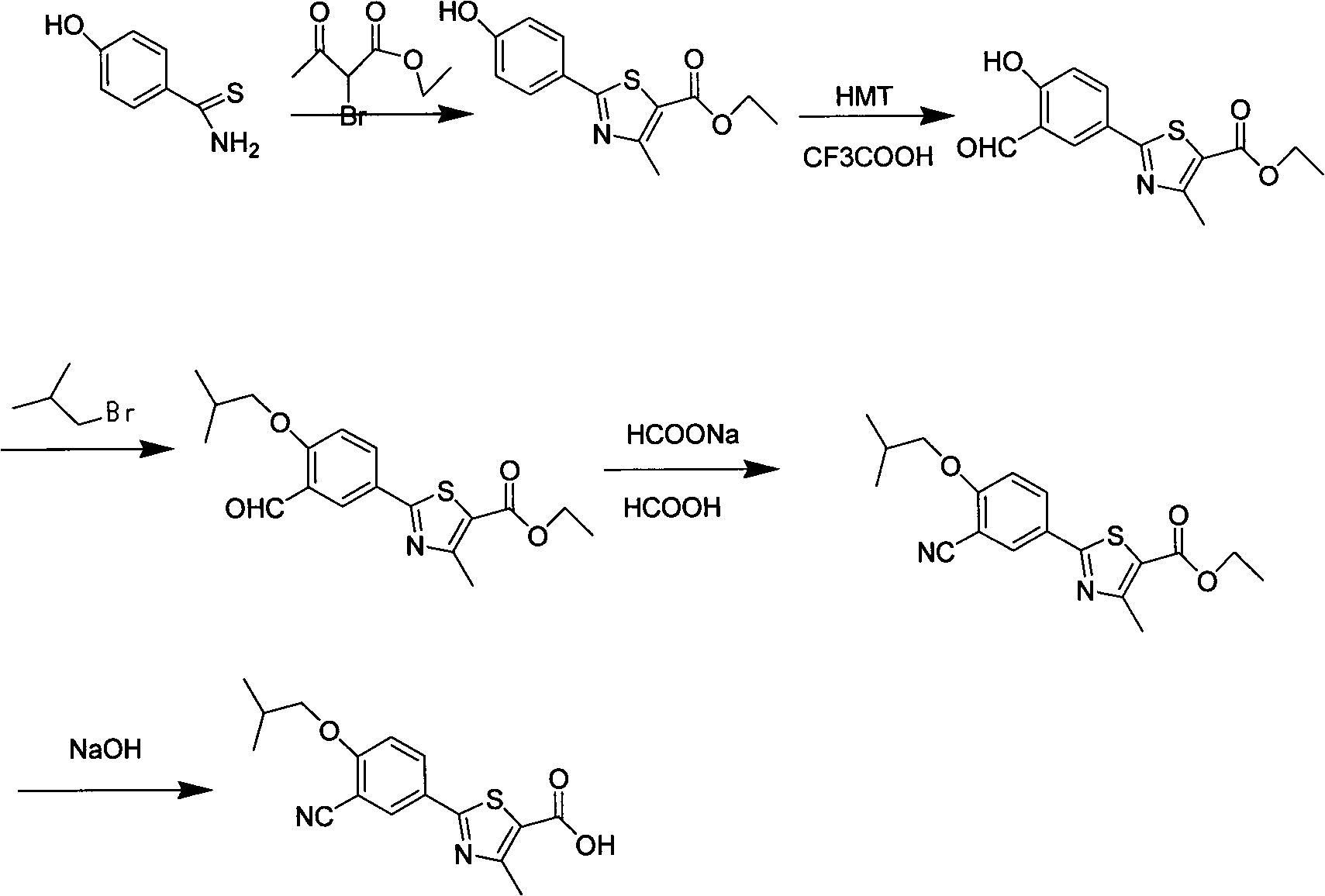
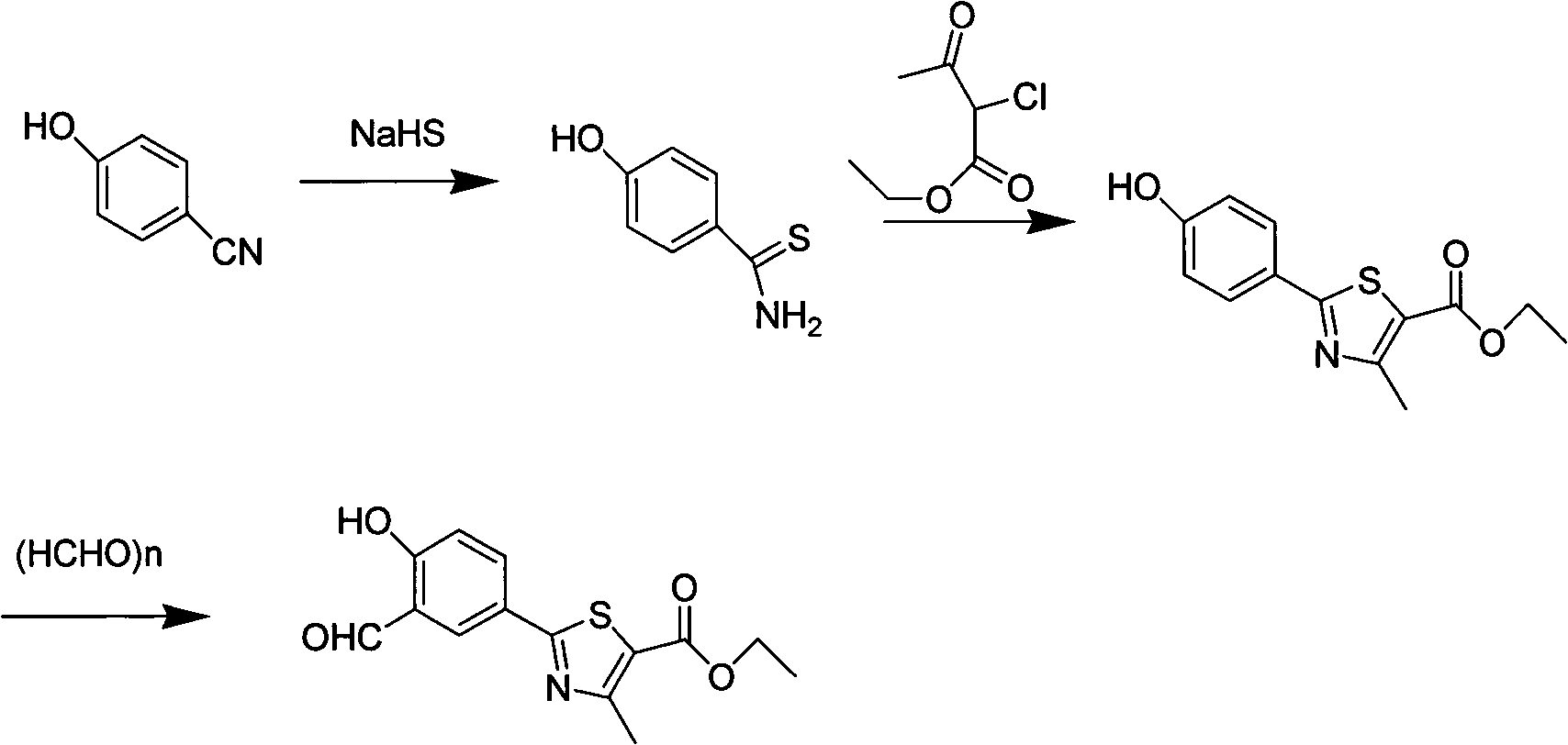
Preparation method of febuxostat intermediate
A technology of hydroxyphenyl and methyl group, applied in the field of medicinal chemistry, can solve the problems such as the large corrosion of trifluoroacetic acid equipment, which is difficult for industrialization, the environmental pollution of hydrogen sulfide, which is unsuitable for industrialization, and the preciousness of hydroxybenzene thiocarboxamide, so as to overcome the equipment corrosion. Powerful, easy to industrialize production, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of p-Hydroxythiobenzamide
[0024] Add 30 g of p-cyanophenol and 500 ml of DMF into a 1 L three-necked flask, stir, add 40 g of 70% sodium hydrosulfide, and react at room temperature for 50 g of magnesium chloride hexahydrate for 24 hours. TLC detection (petroleum ether: ethyl acetate=2: 1) confirms the reaction It was nearly complete, poured into 2L of water under stirring, cooled to 8°C with ice water, extracted with ethyl acetate, spin-dried the solvent and dried to obtain 36.6 g of brown solid, yield 95%, mp.201-202°C.
Embodiment 2
[0026] Synthesis of ethyl 2-(4-hydroxyphenyl)-4-methyl-thiazole-5-carboxylate
[0027] Add 113g of 4-hydroxythiobenzamide and ethanol into a 2L four-necked flask, stir and heat to 60°C, stop heating, add 2-ethyl chloroacetoacetate dropwise, continue to reflux for 4 hours after dropping, TLC detection ( Petroleum ether: ethyl acetate = 2:1) to confirm that the reaction was complete, after cooling, it was filtered with suction, washed with 150 mL of ethanol, and dried at 60°C to obtain 155.7 g of a light yellow solid with a yield of 80%. mp.210~211℃.
Embodiment 3
[0029] Synthesis of 2-(3-formyl-4-hydroxyphenyl)-4-methyl-thiazole-5-carboxylic acid ethyl ester
[0030] Add 155.7g of ethyl 2-(4-hydroxyphenyl)-4-methyl-5-thiazolecarboxylate and an appropriate amount of acetonitrile into a 2L four-necked flask, stir, add 93g of anhydrous magnesium chloride, and add 124g of paraformaldehyde in batches, Add 310mL of triethylamine dropwise, heat to reflux for 6 hours, TLC detection (petroleum ether: ethyl acetate = 2: 1) confirms that the reaction is nearly complete, after cooling slightly, pour an appropriate amount of water to quench the reaction, dilute hydrochloric acid Adjust the pH to 5-6, add a large amount of water, cool and precipitate the product, filter to obtain a yellow solid, and obtain 163.6 g after drying, with a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com