The preparation method of Febuxostat A crystal form
A technology of febuxostat and crystal form, which is applied in the field of medicinal chemistry, can solve the problems of inability to achieve reproducibility, failure to mention the yield and purity of crystal form A, etc., and achieve the effect of easy implementation, high purity and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
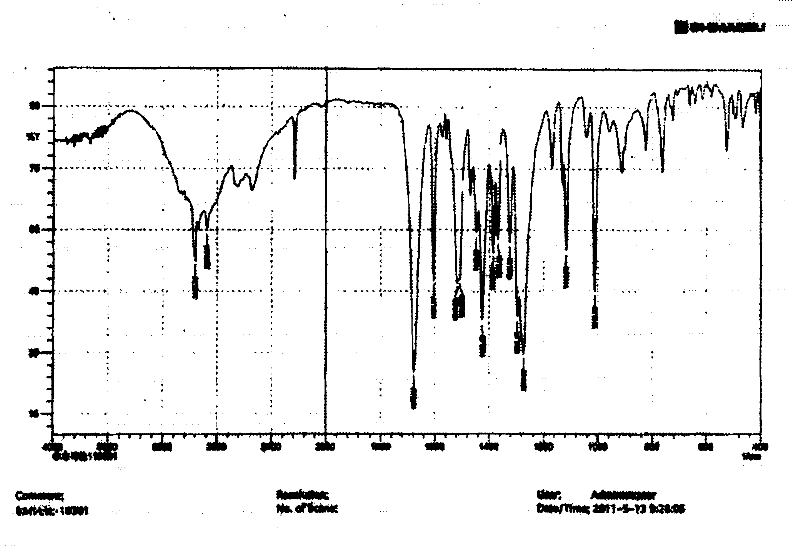
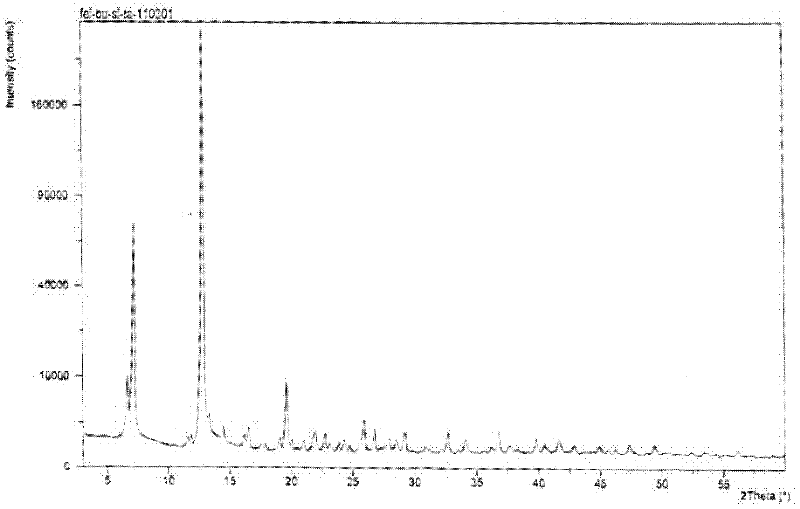
[0027] Put 50g of febuxostat in a 1L three-necked bottle, add 500ml of acetone, heat and reflux in a water bath for 10 minutes, after cooling, place the reaction solution in a constant temperature water bath at 25±2°C, and when crystals start to precipitate, Stirring was continued for 20 minutes at a stirring speed of 600±20 rpm, and then the reaction solution was frozen and crystallized at -10±2°C for 8 hours. Suction. The filter cake was dried under vacuum at 60±2°C and -0.08~-0.10MPa for 6 hours to obtain 47.7g of crystals. Infrared detection, see figure 1 , at 1678cm -1 、1273cm -1 There are characteristic peaks, X-diffraction measurement results see figure 2 , the reflection angle 2θ of the X-ray powder diffraction pattern of the prepared A crystal is 6.64°, 7.22°, 12.86°, 13.33°, 16.54°, 19.63°, 21.99°, 22.74°, 25.91°, 26.76°, 29.22° and have characteristic peaks at 36.74°. Confirmed as febuxostat in A crystal form. The yield was 95.4%, and the purity was 99.98%. ...
Embodiment 2
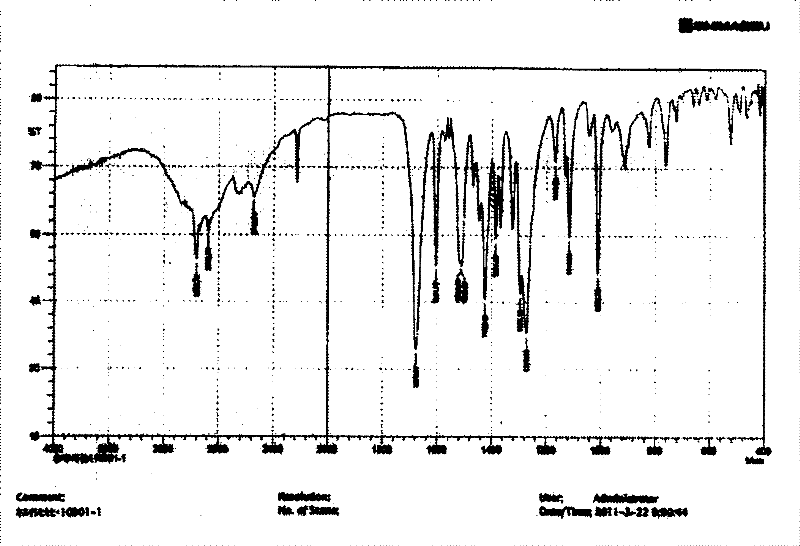
[0029] Put 50g of febuxostat in a 1L three-necked bottle, add 250ml of 95% ethanol, heat and reflux in a water bath for 10 minutes, after cooling, put the reaction solution in a water bath at 30±2°C and let it stand, when crystals begin to precipitate Afterwards, continue to stir for 25 minutes at a stirring speed of 600±20 rpm, and then freeze and crystallize the reaction solution at -12±2°C for 9 hours. Suction. The filter cake was dried under vacuum at 60±2°C and -0.08~-0.10MPa for 7 hours to obtain 47.3g of crystals. Infrared detection, see image 3 , at 1678cm -1 、1274cm -1 There are characteristic peaks, X-diffraction measurement results see Figure 4 , the reflection angle 2θ of the X-ray powder diffraction pattern of the prepared A crystal is 6.63°, 7.22°, 12.84°, 13.31°, 16.54°, 19.62°, 22.00°, 22.74°, 25.92°, 26.74°, 29.22° and have characteristic peaks at 36.75°. Confirmed as febuxostat in A crystal form. The yield was 94.6%, and the purity was 99.96%.
Embodiment 3
[0031] Put 50g of febuxostat in a 1L three-necked bottle, add 750ml of methyl ethyl ketone, heat and reflux in a water bath for 10 minutes, and after cooling, place the reaction solution in a water bath at 35±2°C to stand still. When crystals start to precipitate, Stirring was continued for 30 min at a stirring speed of 600±20 rpm, and then the reaction solution was frozen and crystallized at -8±2°C for 10 hours. Suction. The filter cake was dried under vacuum at 60±2°C and -0.08~-0.10MPa for 8 hours to obtain 46.4g of crystals. Infrared detection, see Figure 5 , at 1678cm -1 、1273cm -1 There are characteristic peaks, X-diffraction measurement results see Figure 6 , the reflection angle 2θ of the X-ray powder diffraction pattern of the prepared A crystal is 6.62°, 7.20°, 12.83°, 13.30°, 16.52°, 19.60°, 21.96°, 22.71°, 25.90°, 26.73°, 29.19° and have characteristic peaks at 36.73°. Confirmed as febuxostat in A crystal form. The yield was 92.8%, and the purity was 99.92...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com