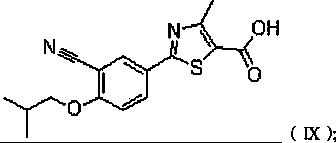
Preparation methods of compound 2-(3-formyl-4-isobutoxy phenyl)-4-methyl thiazole-5-ethyl formate and febuxostat
A technology of isobutoxyphenyl and isobutoxybenzonitrile, which is applied in the field of compound preparation, can solve problems such as low safety, many by-products, and difficult conditions to control, and achieve high safety, high yield, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
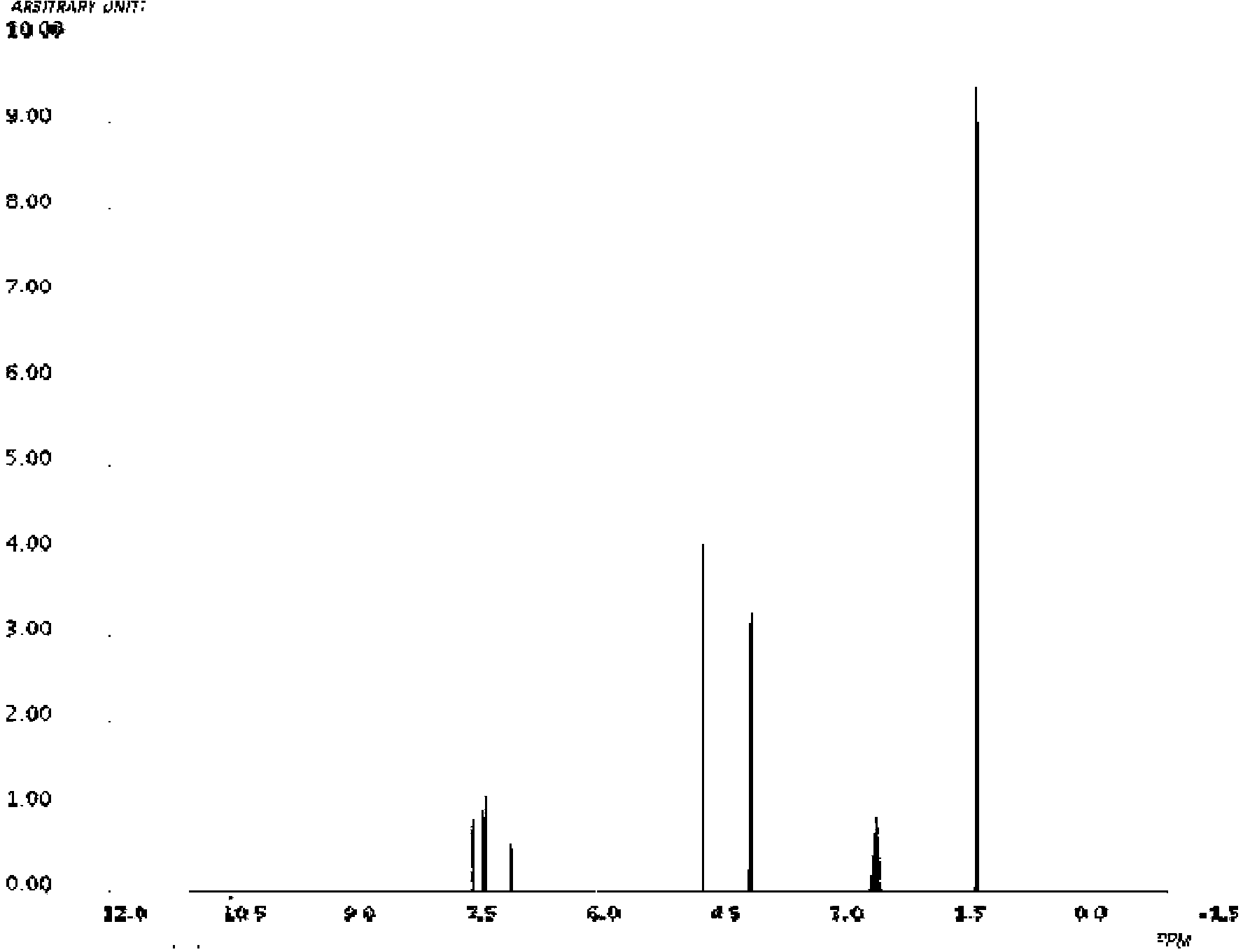
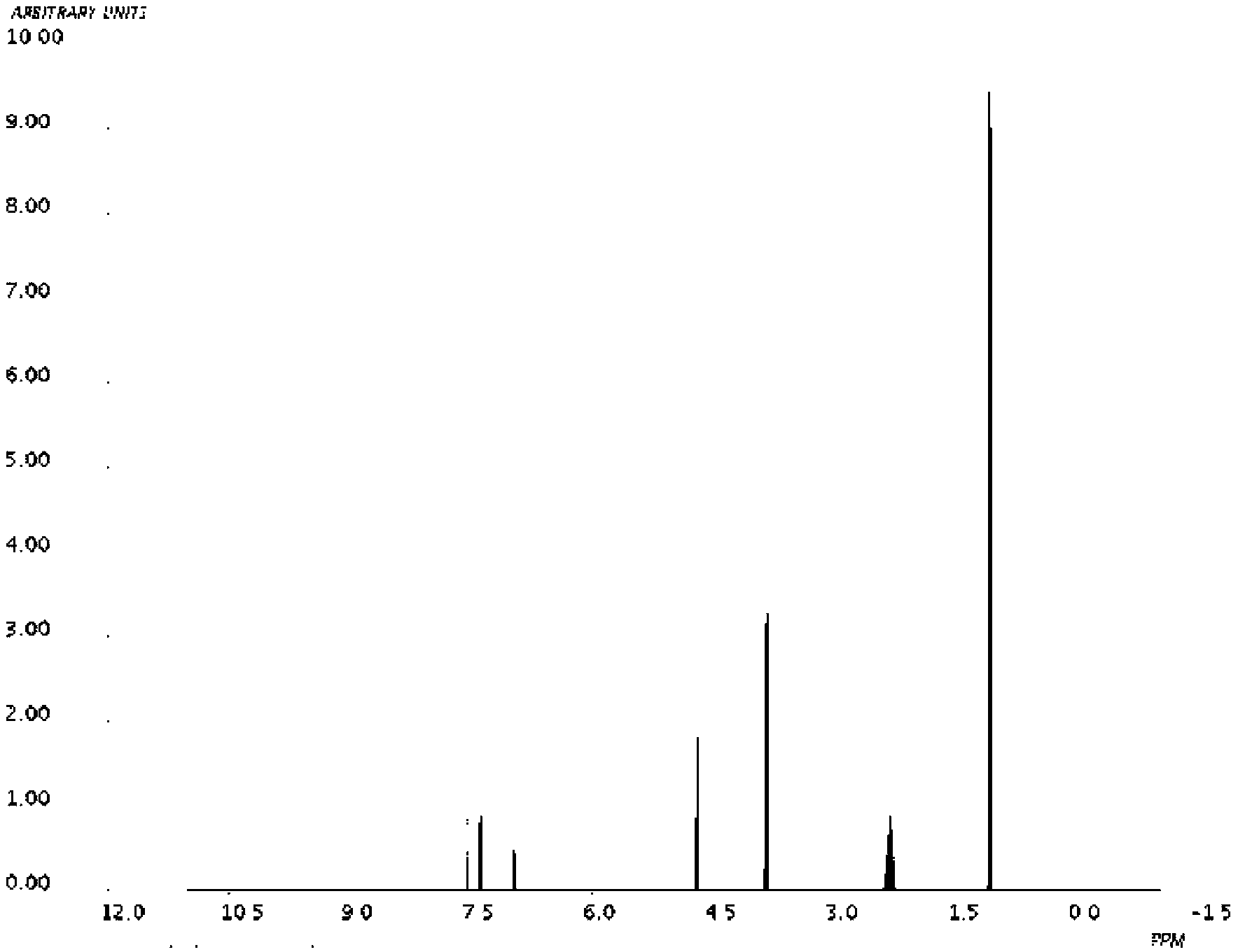
Image
Examples
preparation example Construction
[0040] The embodiment of the present invention discloses a preparation method of ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate, comprising the following steps:
[0041] (A) 4-isobutoxybenzonitrile reacts with a chloromethylation reagent under the action of a catalyst to obtain a compound with the structure of formula (III);
[0042]
[0043] (B) reacting the compound with the structure of formula (III) with water under the action of a catalyst to obtain the compound with the structure of formula (IV);
[0044]
[0045] (C) The compound with the structure of formula (IV) undergoes an oxidation reaction under the action of an oxidizing agent to obtain the compound with the structure of formula (V);
[0046]
[0047] (D) reacting the compound with the structure of formula (V) with sodium hydrosulfide to obtain the compound with the structure of formula (VI);
[0048]
[0049] (E) The compound with the structure of formula (VI) is reacted with et...
Embodiment 1
[0074] Add 59.6g (0.5mol) of p-hydroxybenzonitrile and 1000mL of DMF to a 3000mL four-necked round-bottom flask in sequence, add 276.4g (2.0mol) of anhydrous potassium carbonate, 33.2g (0.2mol) of potassium iodide and 274.0g of (2.0mol) isobutane bromide, slowly warm up to 80°C, stir for 6 hours, stabilize the reaction at this temperature, track with TLC during the reaction, until the raw material is completely converted, after the reaction is completed, suction filter, filter cake with 30mLN, Wash with N-dimethylformamide, add 400 mL of water to the combined filtrate, and extract with methyl tert-butyl ether. The filtrate was dried with anhydrous sodium sulfate, filtered, concentrated under reduced pressure, desolvated and refined with 5-7% ethyl acetate / n-hexane to obtain 84.6g of 4-isobutoxybenzonitrile with a yield of 98.5%, HPLC purity is 98.87%.
[0075] Add 13.9g of 4-isobutoxybenzonitrile, 9.45g of methoxyacetyl chloride and 200mL of nitromethane sequentially into a f...
Embodiment 2
[0081] Add 59.6g (0.5mol) of p-hydroxybenzonitrile and 1000mL of DMF to a 3000mL four-necked round-bottom flask in sequence, add 276.4g (2.0mol) of anhydrous potassium carbonate, 33.2g (0.2mol) of potassium iodide and 274.0g of (2.0mol) isobutane bromide, slowly warm up to 80°C, stir for 6 hours, stabilize the reaction at this temperature, track with TLC during the reaction, until the raw material is completely converted, after the reaction is completed, suction filter, filter cake with 30mLN, Wash with N-dimethylformamide, add 400 mL of water to the combined filtrate, and extract with methyl tert-butyl ether. The filtrate was dried with anhydrous sodium sulfate, filtered, concentrated under reduced pressure, desolvated and refined with 5-7% ethyl acetate / n-hexane to obtain 84.6g of 4-isobutoxybenzonitrile with a yield of 98.5%, HPLC purity is 98.87%.
[0082] Add 13.9g of 4-isobutoxybenzonitrile, 3.0g of paraformaldehyde and 50mL of nitromethane in sequence into a 100mL thre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com