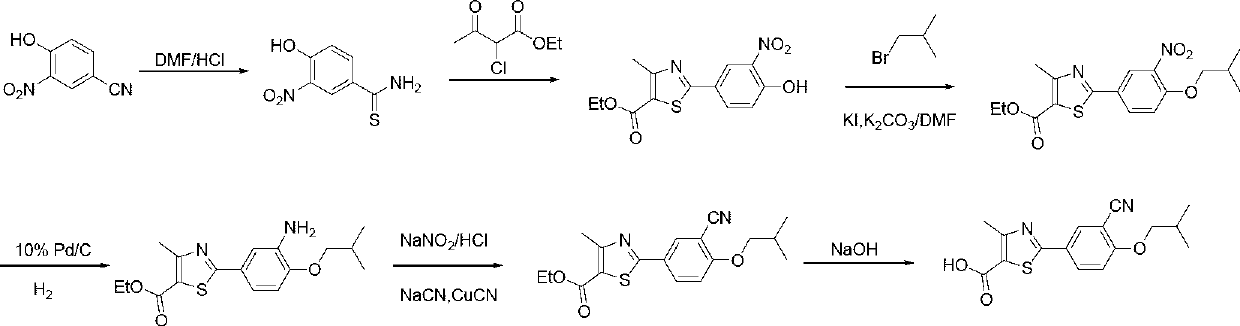
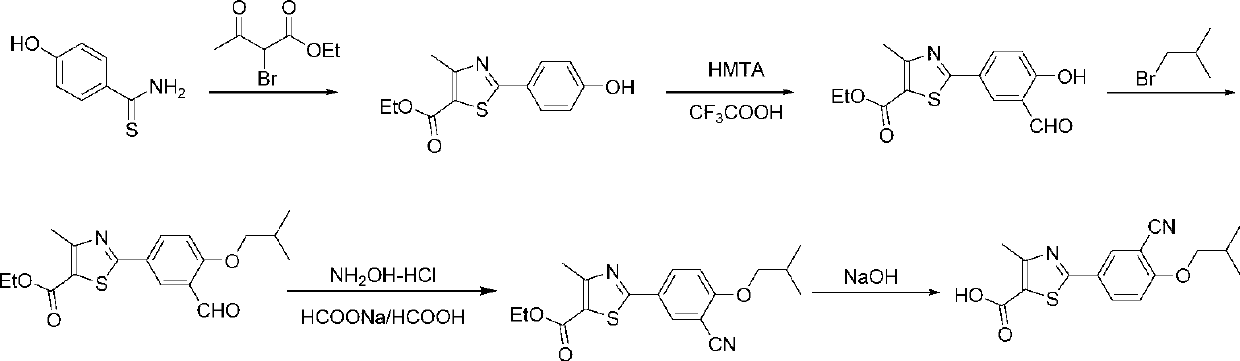
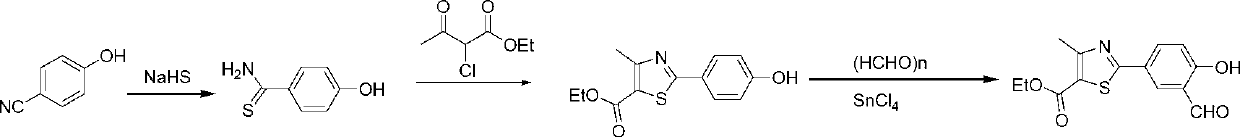
Synthetic method of febuxostat
A synthetic method and febuxostat technology, applied in the field of medicinal chemistry, can solve problems such as difficult preparation, difficult industrialization, heavy environmental pollution, etc., and achieve the effects of easy control of reaction conditions, easy industrial production, and simple experimental operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0051] The preparation of embodiment 14-hydroxyl-1,3-benzenedicarbaldehyde:
[0052]
[0053] In the three-necked flask, add 64mL 36% (0.84mol) formaldehyde aqueous solution, 82mL (0.79mol) salicylaldehyde and 500mL concentrated hydrochloric acid, under cooling and stirring, feed HCl (g), keep the reaction temperature at 15~20°C, about A white solid appeared in 1h, and HCl (g) was continued for 2.5h to stop the reaction. After standing for 2h, filter with suction, and wash the filter cake with water 3 times. The crude product was dissolved in ethyl acetate, washed with 10% sodium bicarbonate solution, and washed with water until neutral. After water separation, the ethyl acetate layer was dried over anhydrous sodium sulfate. Evaporate most of the solvent under reduced pressure, add petroleum ether (60-90°C) to recrystallize the residue after cooling, and obtain 99.1 g of white needle-like crystals of 5-chloromethyl salicylaldehyde, yield 73.3%, mp 84-86 ℃ (literature val...
Embodiment 24
[0056] The preparation of embodiment 24-hydroxyl-1,3-dibenzonitrile:
[0057]
[0058] In a 1L round bottom flask, 47.0 g (0.33 mol) of 4-hydroxy-1,3-phthalaldehyde was dissolved in acetonitrile (470 mL). Add 28.0g (0.40mol, 1.2eq) of hydroxylamine hydrochloride to it at a controlled temperature of 15-25°C, stir at 15-25°C for 0.5h, then raise the temperature to reflux temperature, and maintain the temperature for 5h to obtain a light yellow solution , TLC detected that the reaction was complete. Most of the solvent was removed under reduced pressure and the temperature was lowered. The residue was diluted with ethyl acetate (100 mL) and poured into ice water. The organic phase was separated, and the aqueous phase was extracted with ethyl acetate (300 mL×2). The organic phases were combined, washed successively with water and saturated brine, and dried over anhydrous sodium sulfate. After removing the solvent under reduced pressure, a yellow solid was obtained, and the cr...
Embodiment 34
[0059] Preparation of Example 34-isobutoxy-1,3-dibenzonitrile:
[0060]
[0061] In a 1L round bottom flask, 41.8 g (0.29 mol) of 4-hydroxy-1,3-phthalonitrile was dissolved in acetonitrile (490 mL). Potassium carbonate 84g (0.61mol, 2.1eq) and potassium iodide (200mg) were added thereto at a controlled temperature of 15-25°C. After the addition was complete, the mixture was slowly heated to reflux temperature. After keeping the reflux for 0.5h, a solution of 43.7g (34.7mL, 0.32mol, 1.1eq) of bromoisobutane in acetonitrile (30mL) was slowly added dropwise, and the reaction was carried out under reflux for 9h. Most of the solvent was distilled off under reduced pressure and the temperature was lowered. The residue was dissolved in ethyl acetate (150 mL) and the insoluble matter was removed by suction filtration. The mother liquor was diluted with ethyl acetate (300 mL), washed with water three times, then with saturated brine, and finally dried over anhydrous sodium sulfate....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com