Medicine compound containing febuxostat
A technology of febuxostat and composition, applied in the field of pharmaceutical composition containing febuxostat, capable of solving problems such as inability to obtain dissolution curve deviation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
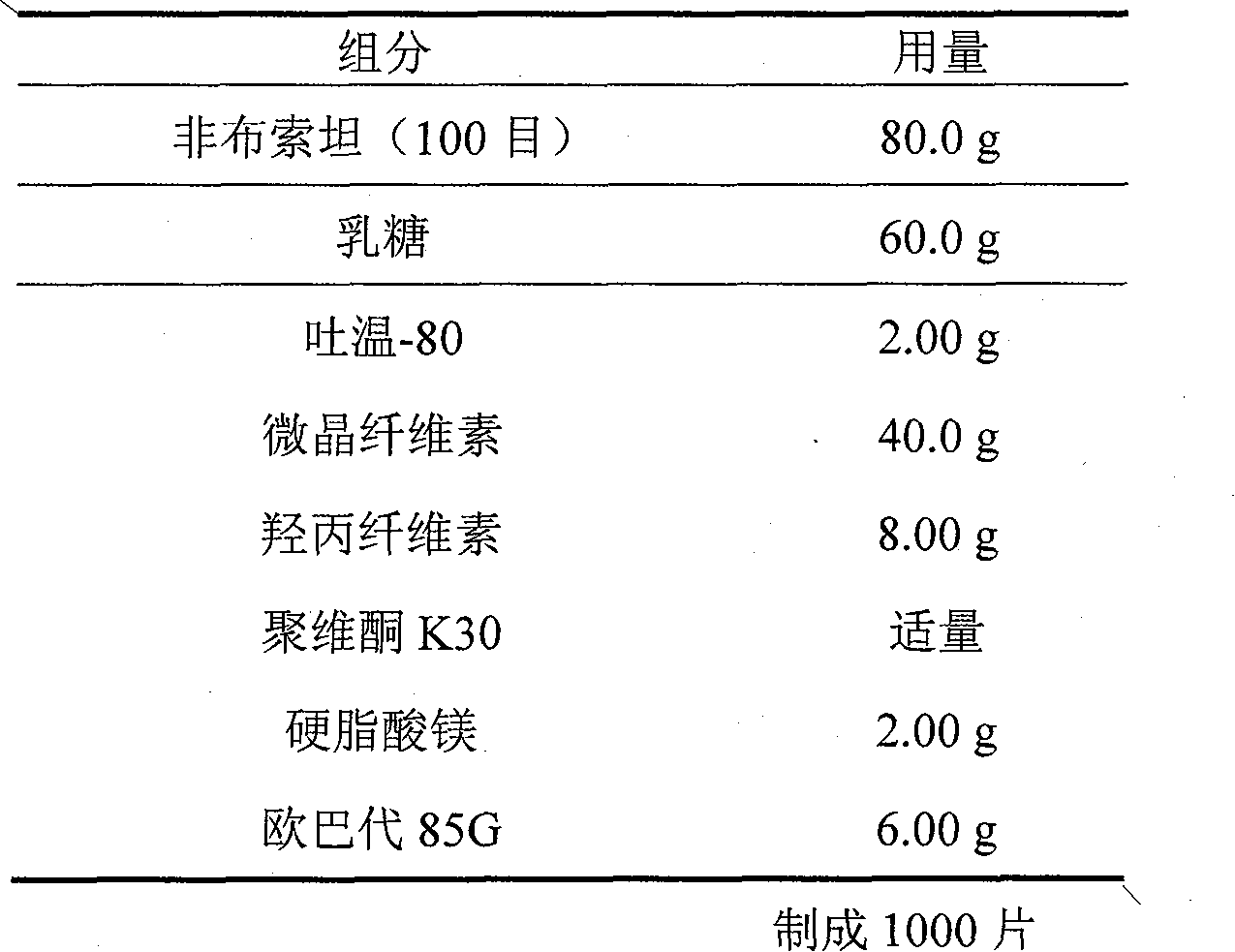
Embodiment 1
[0017]
[0018] Preparation:
[0019] Dissolve povidone K30 and Tween-80 in a certain amount of ethanol as a binder; mix febuxostat with lactose and microcrystalline cellulose hypromellose, and add the above binder, Stir the formulation; dry at 40-60°C to obtain dry granules; granulate the dry granules with a granulator, add magnesium stearate, compress into tablets, coat with 15% Opadry water dispersion, pack, Instantly.
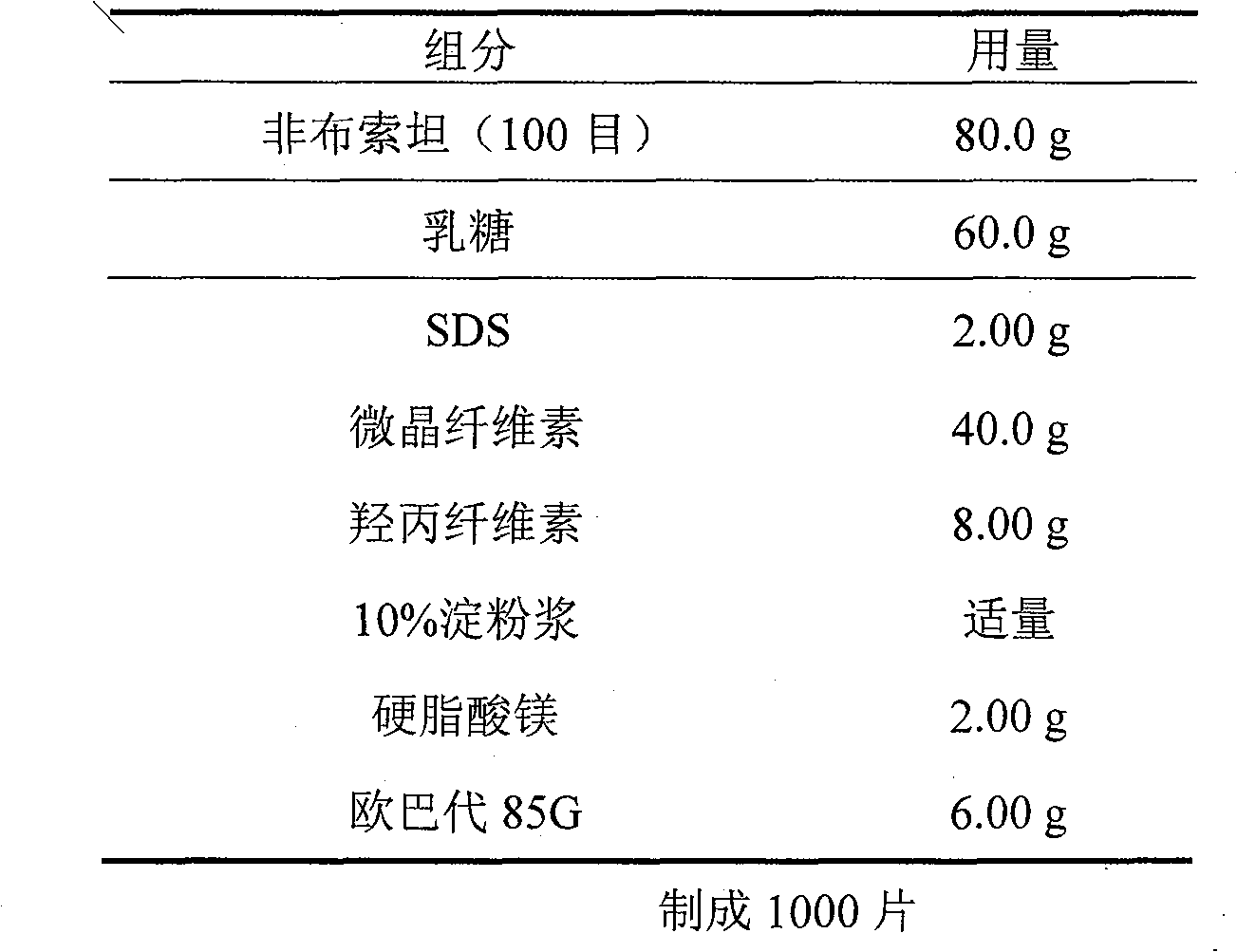
Embodiment 2
[0021]
[0022] Preparation:
[0023] Dissolve SDS in a certain amount of hot water, add starch to make 10% starch slurry as a binder; mix febuxostat with lactose, microcrystalline cellulose, and hydroxypropyl cellulose, and add the above Adhesive, stirring preparation; drying at 40-60°C to obtain dry granules; granulating the dry granules, adding magnesium stearate, tableting, coating with 15% Opadry water dispersion, packaging, that is have to.
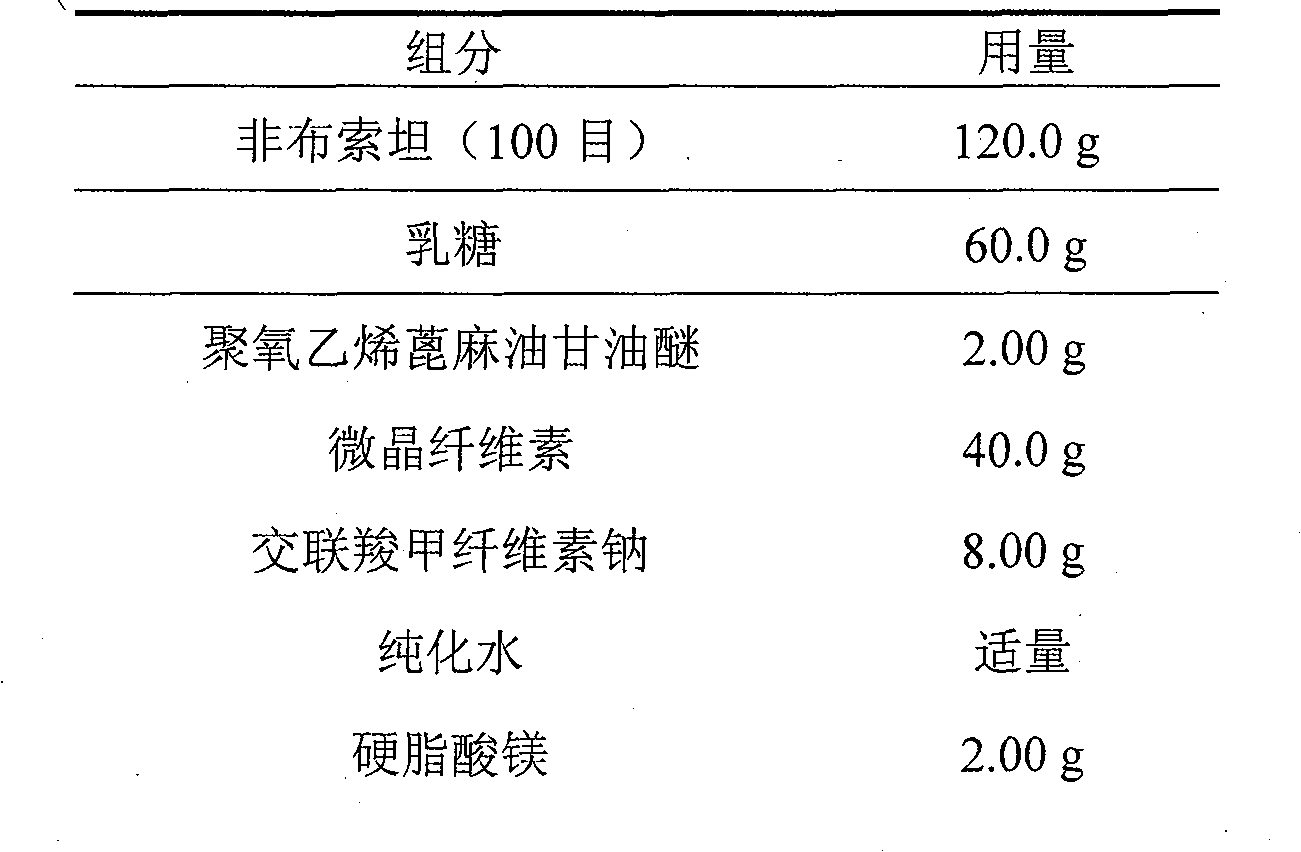
Embodiment 3
[0025]
[0026]
[0027] Preparation:
[0028] Dissolve polyoxyethylene castor oil glyceryl ether (Cremophore EL) in a certain amount of purified water as a wetting agent; mix the prescribed amount of febuxostat with lactose, microcrystalline cellulose, and cross-linked carmellose sodium Then add the above wetting agent, stir the preparation; dry at 40-60°C to obtain dry granules; granulate the dry granules, add magnesium stearate, compress into tablets, and coat with 15% Opadry water dispersion. Pack and serve.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com