Crystal of febuxostat, preparation method and application in medicaments
A technology of febuxostat and crystal, which is applied to the field of febuxostat crystal, preparation and application in medicine, and can solve problems such as unsatisfactory dissolution results and need to be improved, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 10g of febuxostat and 500ml of toluene into a 1L round-bottomed flask, heat and stir at 90-100°C until the solids are completely dissolved, then cool naturally to room temperature, a large amount of crystals will precipitate out quickly, in order to increase the yield, continue to cool in an ice bath To -5 ~ 0 ° C, heat preservation, stirring and crystallization for 2 hours, filter, wash the filter cake with a small amount of toluene, and dry it in vacuum at 80 ° C for 8 hours to obtain 7.8 g of light yellow crystalline powder with a purity of ≥ 99% (HPLC. Normalization method ).
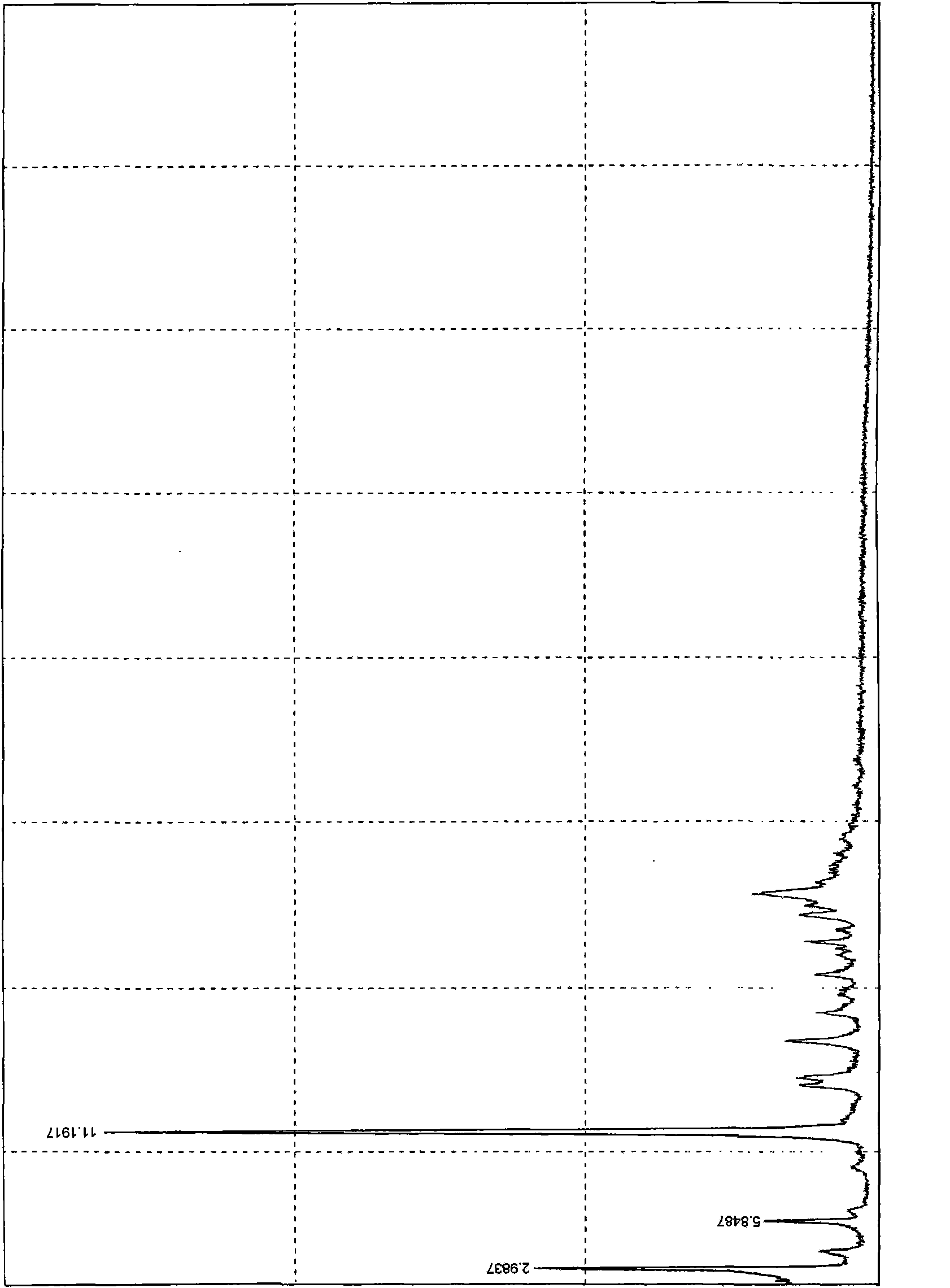
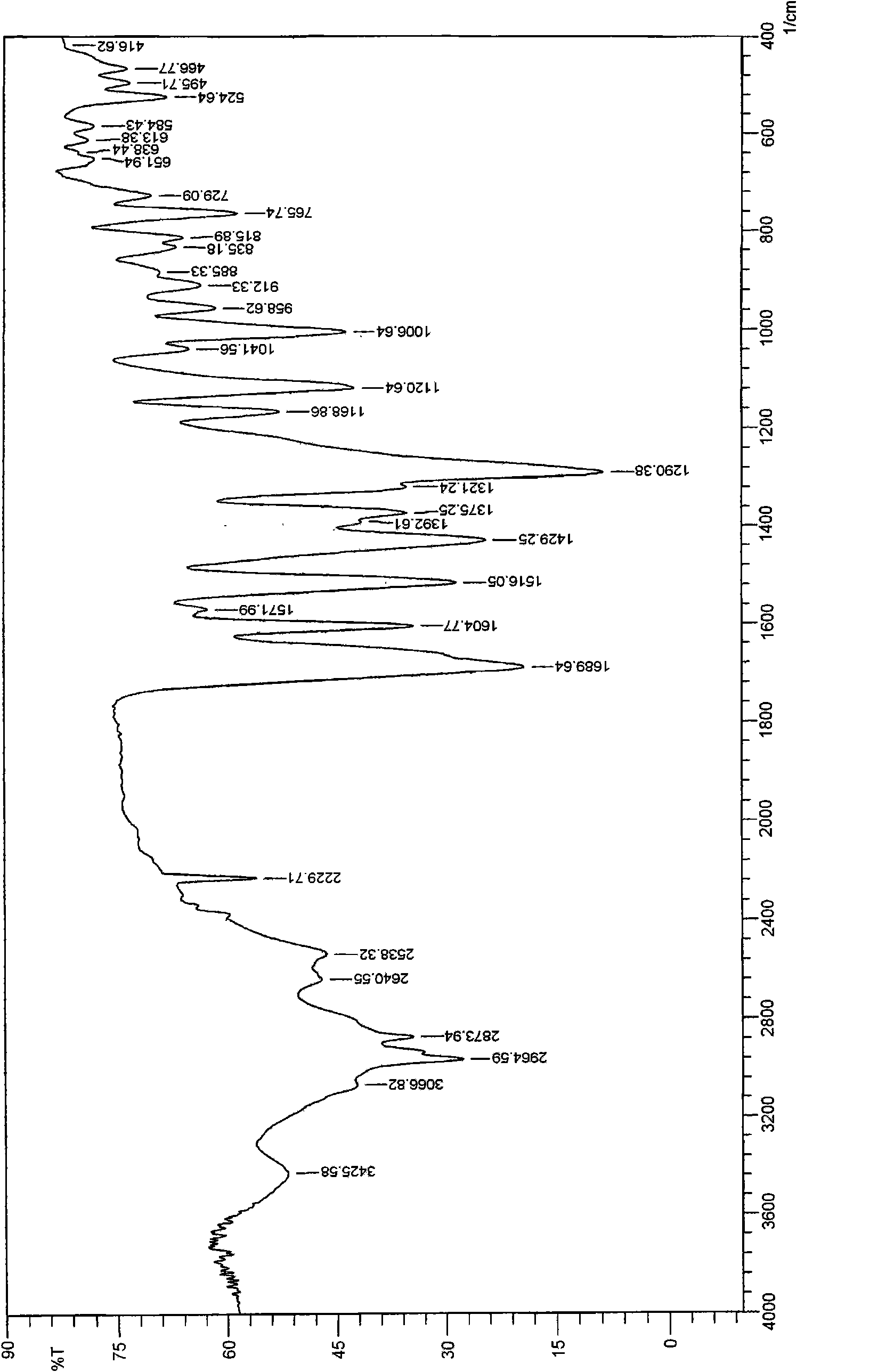
[0020] Sampling to determine the reflection angle 2θ absorption peak of its X-ray powder diffraction is as follows: figure 1 Shown, the data of absorption peak is shown in Table 1; Figure 2 ~ Figure 3 shown. Depend on figure 1 As can be seen from Table 1, the reflection angle 2θ of the crystal X-ray powder diffraction pattern is about 2.98°, 5.84°, 11.19°, 14.04°, 14.54°, 16.75°, 18.4...
Embodiment 2
[0022] Add 10g of febuxostat and 20ml of toluene into a 250ml round-bottomed flask, stir and raise the temperature to reflux. After all the solids are dissolved, cool naturally to room temperature (15-30°C). Yield: keep stirring and crystallize for 2 hours, filter, wash the filter cake with a small amount of toluene, and dry at 80°C for 8 hours in vacuum to obtain 8.8 g of light yellow crystalline powder with a purity of ≥99% (HPLC. Normalization method). Corresponding X-ray powder diffraction patterns, infrared spectrograms and other detections show that it is a microcrystalline state that also conforms to the relevant characteristics of the M-type crystals mentioned in the present invention.
Embodiment 3
[0024] Prescription: febuxostat M type microcrystal 406g prepared in embodiment 1,
[0025] Medicinal lactose 900g,
[0026] Pregelatinized starch 300g,
[0027] Sodium starch glycolate 120g,
[0029] Mix all components except magnesium stearate in the prescription raw materials evenly, use an appropriate amount of 75% ethanol solution as a binder to make a soft material, extrude through a 14-mesh sieve to make wet granules, and place it in a ventilated and dried state at 80°C for 2 hour, the dry granules are granulated through a 14 mesh sieve, add the remaining magnesium stearate, mix uniformly, fill in No. 2 gelatin capsules, and make 1000 capsule medicines altogether.
[0030] Table 1 Crystal X-ray powder diffraction reflection angle 2θ absorption peak data
[0031]
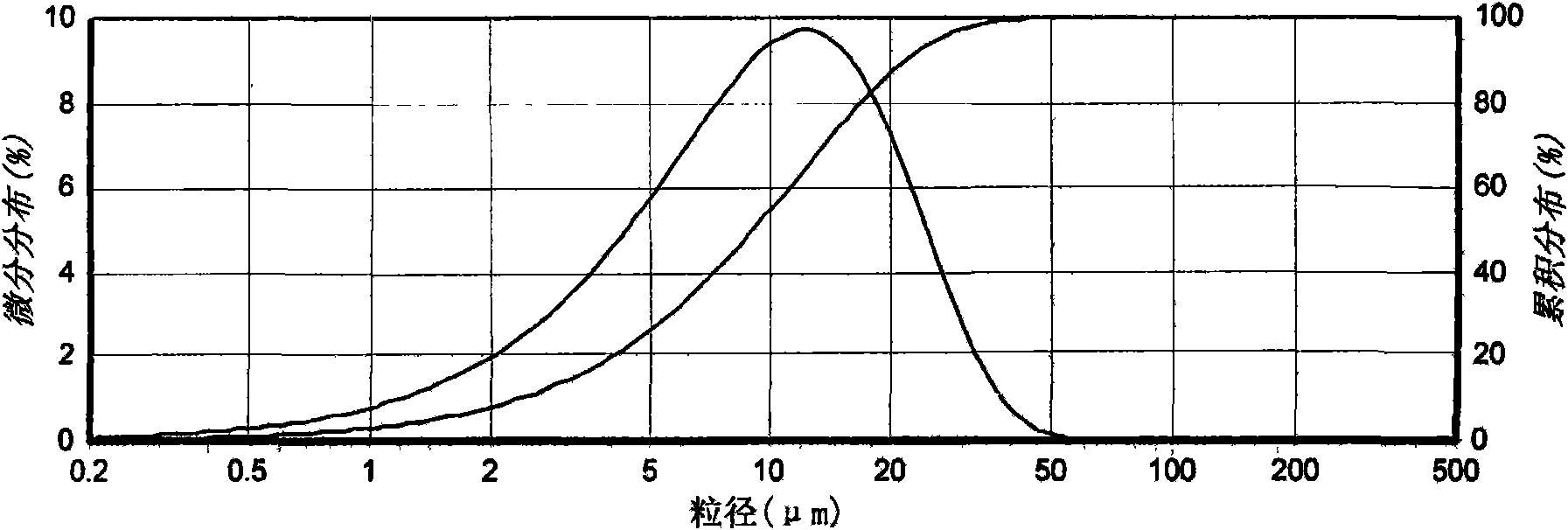
[0032] The detection result of table 2 particle size distribution
[0033...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com