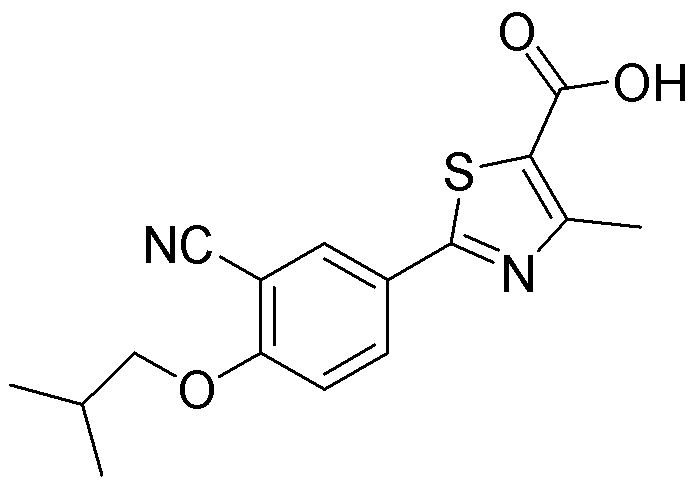
Preparation method of high-purity febuxostat
A febuxostat, high-purity technology, applied in the field of preparation of high-purity febuxostat, can solve problems such as difficult to remove and difficult to achieve product quality, and achieve quality safety, short reaction time, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
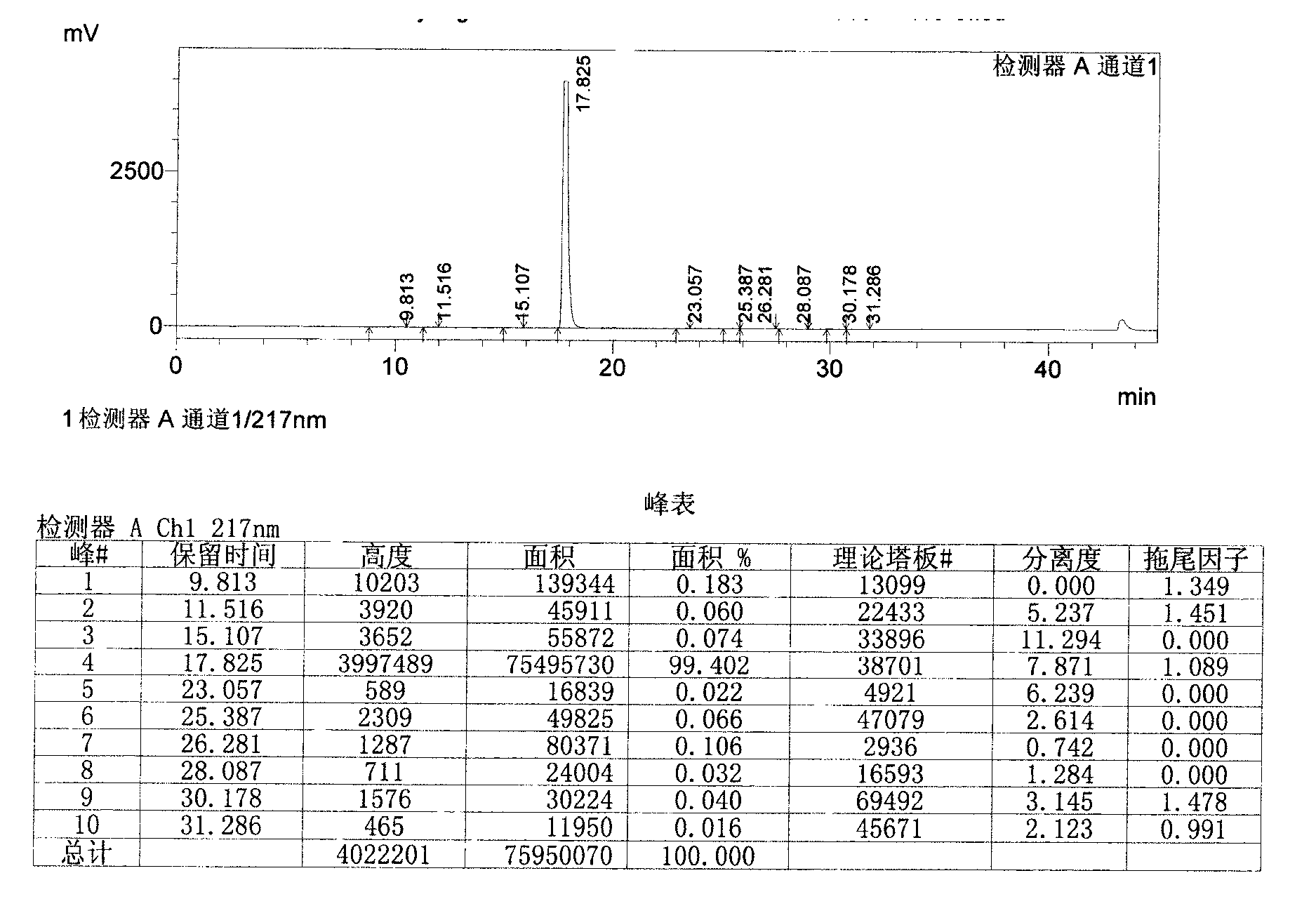
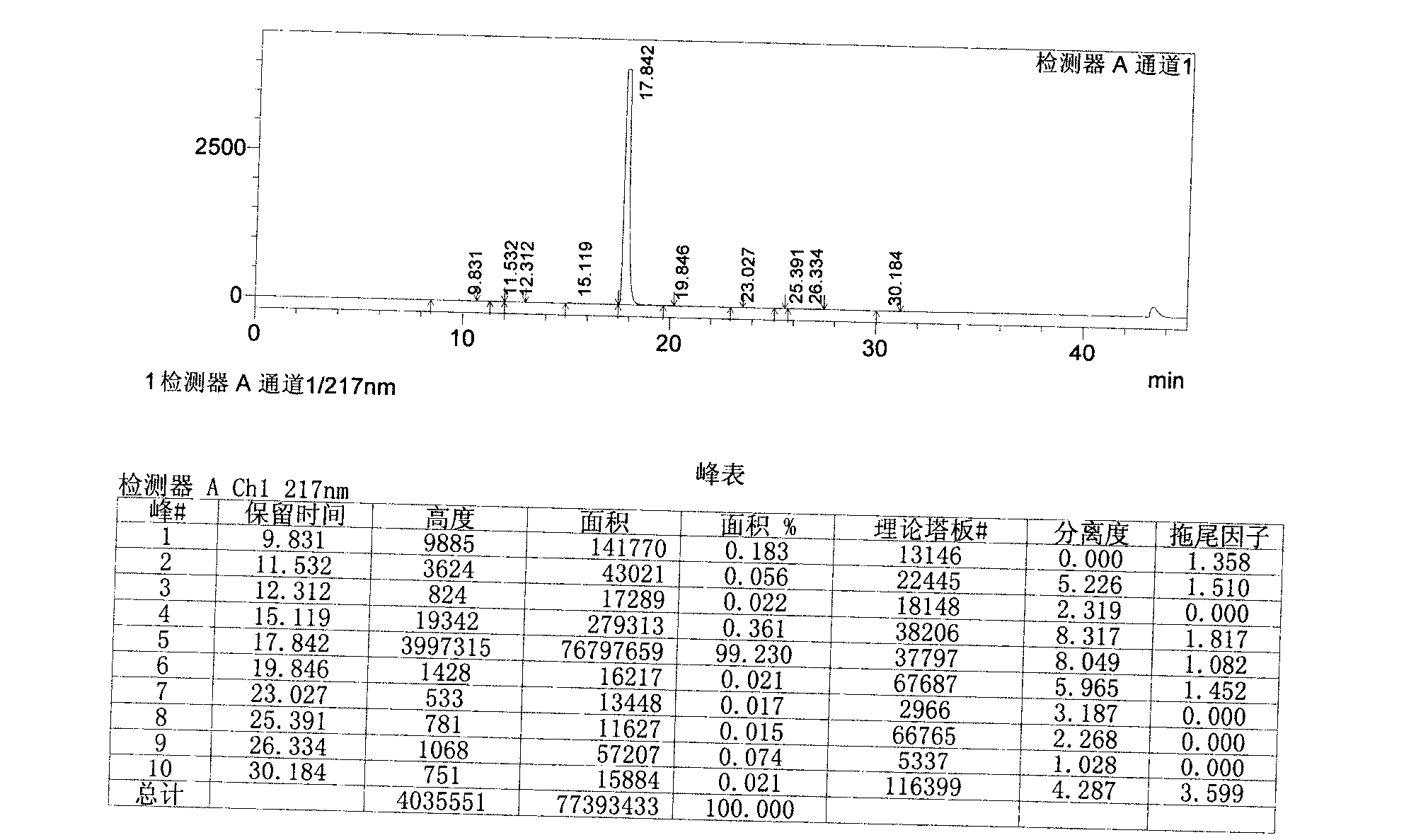
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate
[0049] 1.1 Add 284g of ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate, 200g of potassium carbonate, 1300ml of DMF, 64.3g of potassium iodide into a 5L reaction flask, stir, and heat up to Slowly add bromoisobutane 322g dropwise at 70-80°C, after the dropwise addition is complete, after reacting at 70-80°C for 20 hours, take a sample for TLC (sample is diluted with DMF, developing phase: ethyl acetate:petroleum ether=1:3; Stationary phase: GF254) to monitor the end of the reaction, cool to room temperature, add the reaction solution to 3.8L water, stir for 1 hour, and filter with suction to obtain 2-(3-formyl-4-isobutoxyphenyl)-4-methanol Ethylthiazole-5-carboxylate.
[0050] 1.2 Use distilled bromoisobutane (1-bromopropane content is less than 0.10%) to prepare 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester. Distillation co...
Embodiment 2
[0051] Embodiment 2: Preparation of febuxostat
[0052] Add 274g ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate and 1180ml pyrrolidone in a 5L reaction flask, add 162 g hydroxylamine hydrochloride, 527 g Na 2 SO 4 Heat to 130-140°C, keep for 24 hours, sample HPLC to monitor the reaction end point, after the reaction of the raw materials, cool to room temperature, put the reaction solution into 4L water, stir at room temperature for 1 hour, and filter with suction to obtain 2-(3-cyanide Crude ethyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate.
[0053] Add 200g ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate, 0.4LTHF and 0.4L ethanol, 0.8L1N sodium hydroxide solution into a 5L reaction flask, Stir and react at 30-35°C for 3 hours, sample TLC (developing phase: ethyl acetate: petroleum ether = 1:3; stationary phase: GF254) to monitor the end of the reaction, after the reaction of the raw materials is completed, the reaction liquid i...
Embodiment 3
[0054] Embodiment 3: the refining of febuxostat
[0055] Add 1.9L of acetone to the above crude product, reflux 4.3g of activated carbon and stir for 1h, filter while hot, stir and cool the filtrate below 15°C, filter with suction, add 1.2L of acetone to the obtained wet product, heat to reflux, stir for 0.5h, and cool to Stir at 25°C for 1 hour, filter with suction, and air-dry the obtained product at 65°C for 24 hours to obtain the finished febuxostat product with a yield of 63%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com