Method for determining impurities in febuxostat and its preparation through high performance liquid chromatography
A technology of high-performance liquid chromatography and febuxostat, which is applied in the field of high-performance liquid chromatography to determine impurities in febuxostat and its preparations, can solve the problems of unsuitable analysis, unspecified, unsuitable detection process impurities and degradation Impurities and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
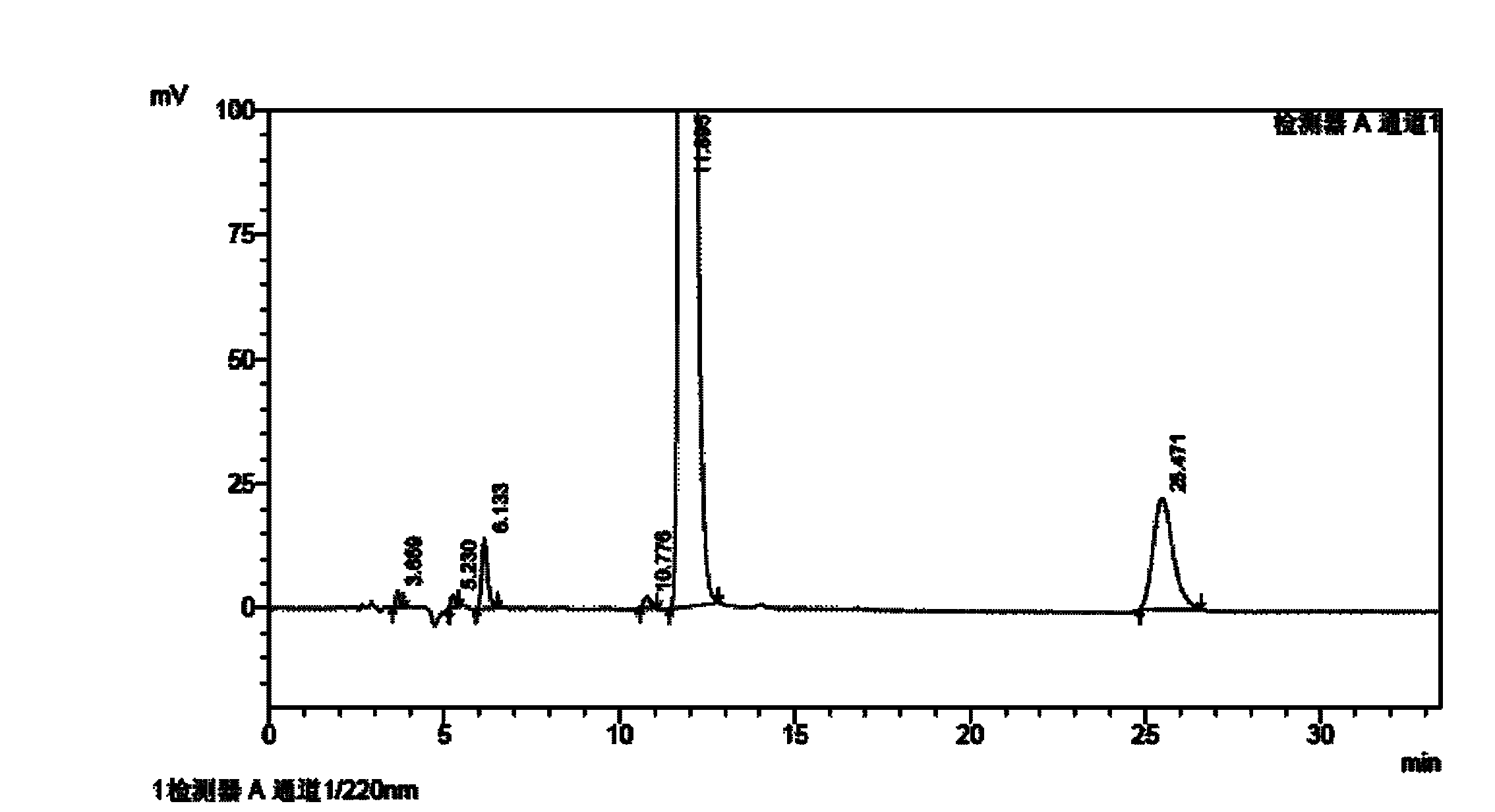
[0092] Homemade febuxostat crude as raw material
[0093] Chromatographic conditions: chromatographic column with octadecylsilane bonded silica gel as filler; 0.01% triethylamine buffer solution as A mobile phase, methanol as B mobile phase, wherein the ratio range of A mobile phase to B mobile phase is 10: 90; detection wavelength 220nm; column temperature 30°C.
[0094] Preparation of impurity reference solution: take p-nitrobenzonitrile, 4-isobutoxy-1,3-benzenedicarbonitrile, 3-cyano-4-isobutoxythiobenzamide, 2- [3-cyano-4-isobutoxyphenyl]-4-methylthiazole-5-ethyl carboxylate, 4-isobutoxy-1,3-bis(4-methylthiazole-5-carboxylate Acid-2-yl)benzene, 2-[3 cyano-4-(2-methylpropoxy)phenyl]-4-methylthiazole, 2-[3-formamido-4-(2- Methylpropoxy)phenyl]-4-methylthiazole-5-carboxylic acid reference substance into a measuring bottle, add mobile phase to dilute to the mark, shake well as impurity reference solution.
[0095] Preparation of the test solution: Accurately weigh an approp...
Embodiment 2
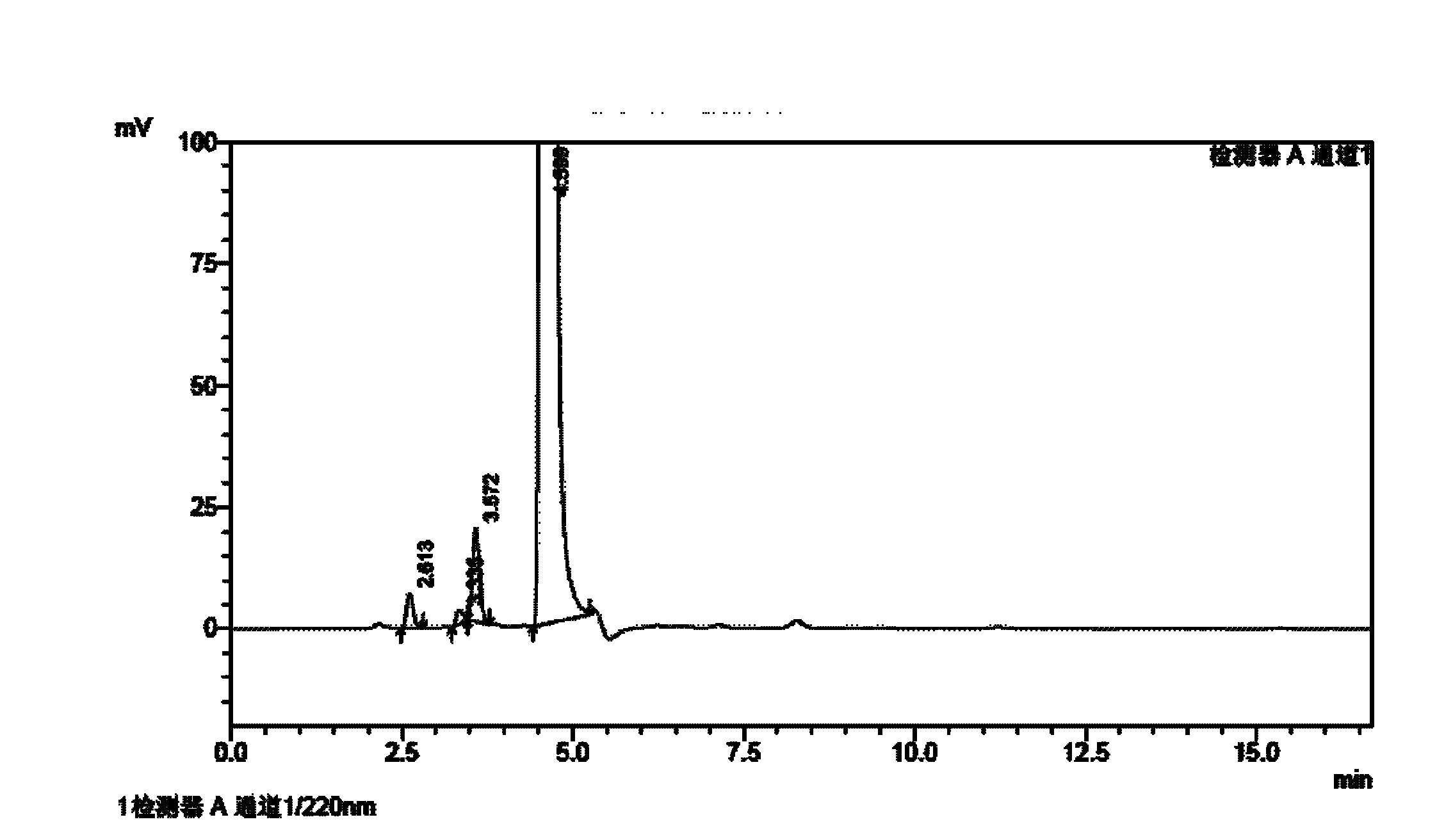
[0100] Homemade febuxostat crude as raw material
[0101] Chromatographic conditions: octadecylsilane bonded silica gel as a chromatographic column; 0.05% triethylamine buffer solution is A mobile phase, methanol is B mobile phase, and the ratio range of A mobile phase to B mobile phase is 25: 75; detection wavelength 317nm; column temperature 30°C.
[0102] Preparation of impurity reference solution: take p-nitrobenzonitrile, 4-isobutoxy-1,3-benzenedicarbonitrile, 3-cyano-4-isobutoxythiobenzamide, 2- [3-cyano-4-isobutoxyphenyl]-4-methylthiazole-5-ethyl carboxylate, 4-isobutoxy-1,3-bis(4-methylthiazole-5-carboxylate Acid-2-yl)benzene, 2-[3 cyano-4-(2-methylpropoxy)phenyl]-4-methylthiazole, 2-[3-formamido-4-(2- Methylpropoxy)phenyl]-4-methylthiazole-5-carboxylic acid reference substance into a measuring bottle, add mobile phase to dilute to the mark, shake well as impurity reference solution.
[0103] Preparation of the test solution: Accurately weigh an appropriate amount o...
Embodiment 3
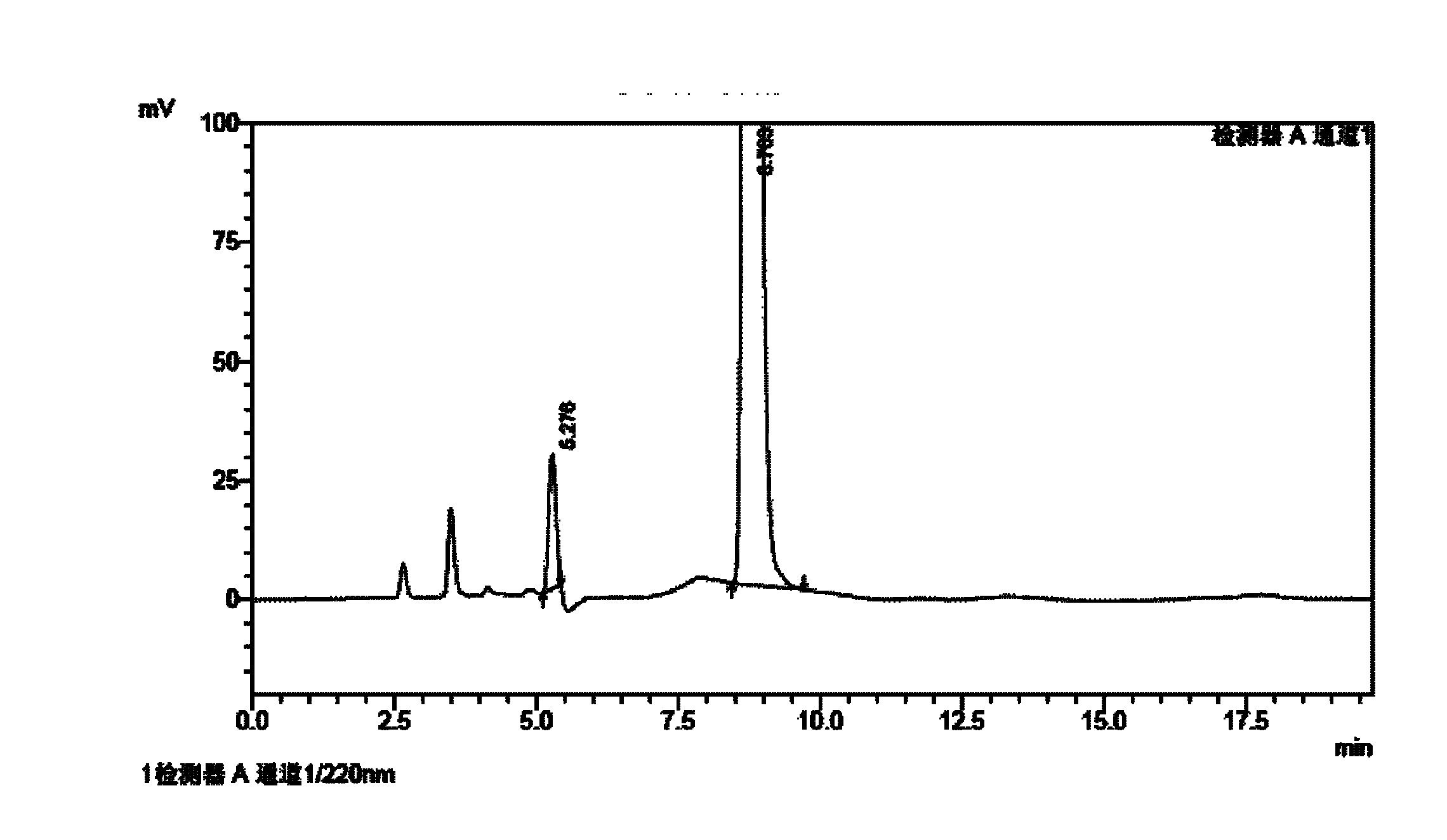
[0108] Homemade febuxostat crude as raw material
[0109] Chromatographic conditions: chromatographic column with octadecylsilane bonded silica gel as filler; 0.10% triethylamine buffer solution as A mobile phase, methanol as B mobile phase, wherein the ratio range of A mobile phase to B mobile phase is 40: 60; detection wavelength 310nm; column temperature 30°C.
[0110] Preparation of impurity reference solution: take p-nitrobenzonitrile, 4-isobutoxy-1,3-benzenedicarbonitrile, 3-cyano-4-isobutoxythiobenzamide, 2- [3-cyano-4-isobutoxyphenyl]-4-methylthiazole-5-ethyl carboxylate, 4-isobutoxy-1,3-bis(4-methylthiazole-5-carboxylate Acid-2-yl)benzene, 2-[3 cyano-4-(2-methylpropoxy)phenyl]-4-methylthiazole, 2-[3-formamido-4-(2- Methylpropoxy)phenyl]-4-methylthiazole-5-carboxylic acid reference substance into a measuring bottle, add mobile phase to dilute to the mark, shake well as impurity reference solution.
[0111] Preparation of the test solution: Accurately weigh an approp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com