Canagliflozin of crystal form B, and crystallization preparation method thereof
A canagliflozin and crystal form technology, which is applied in the fine chemical industry and application fields, can solve the problems of many steps in the crystallization method, and achieve the effects of low cost, short time consumption, and easy pulverization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
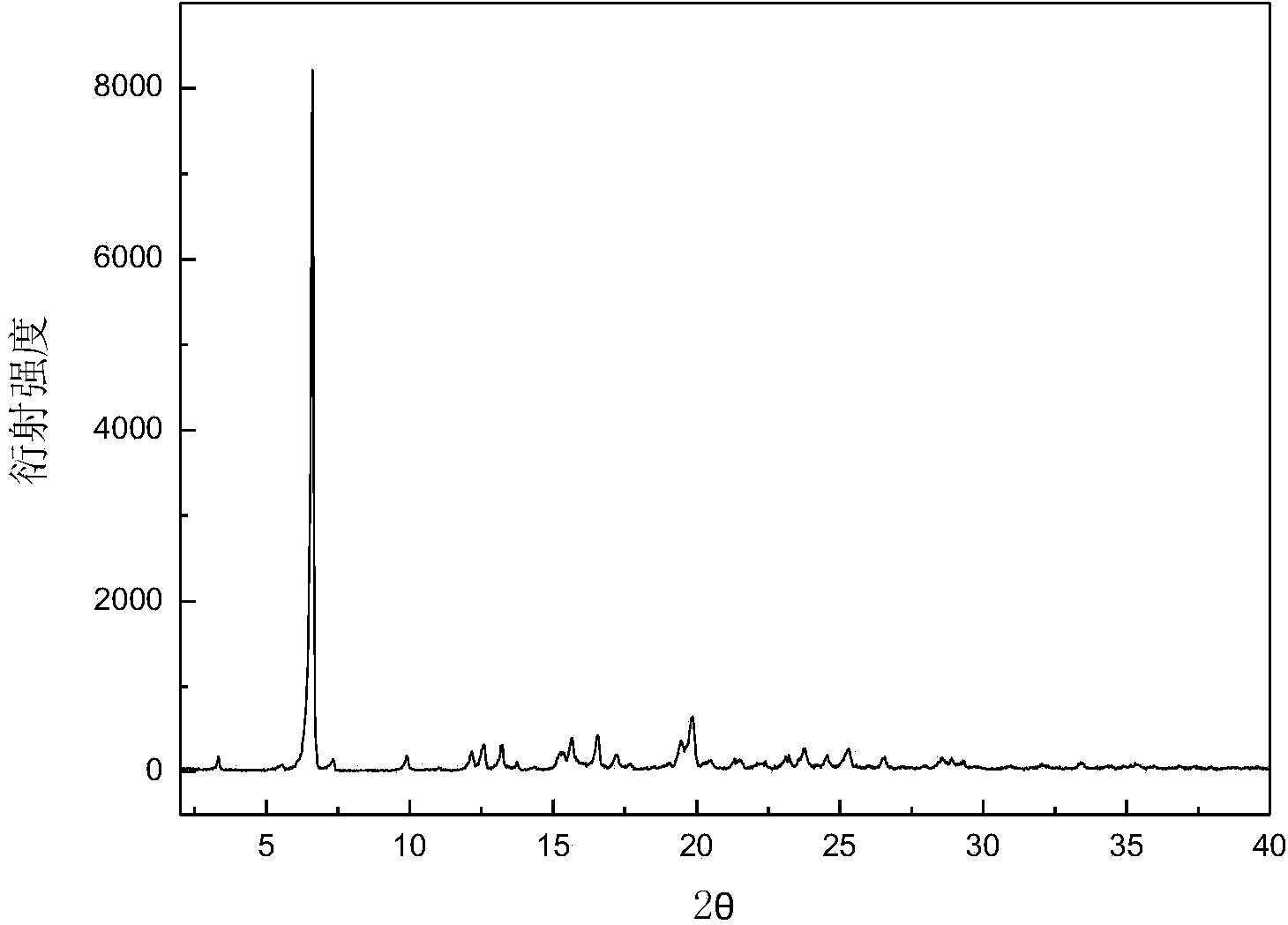
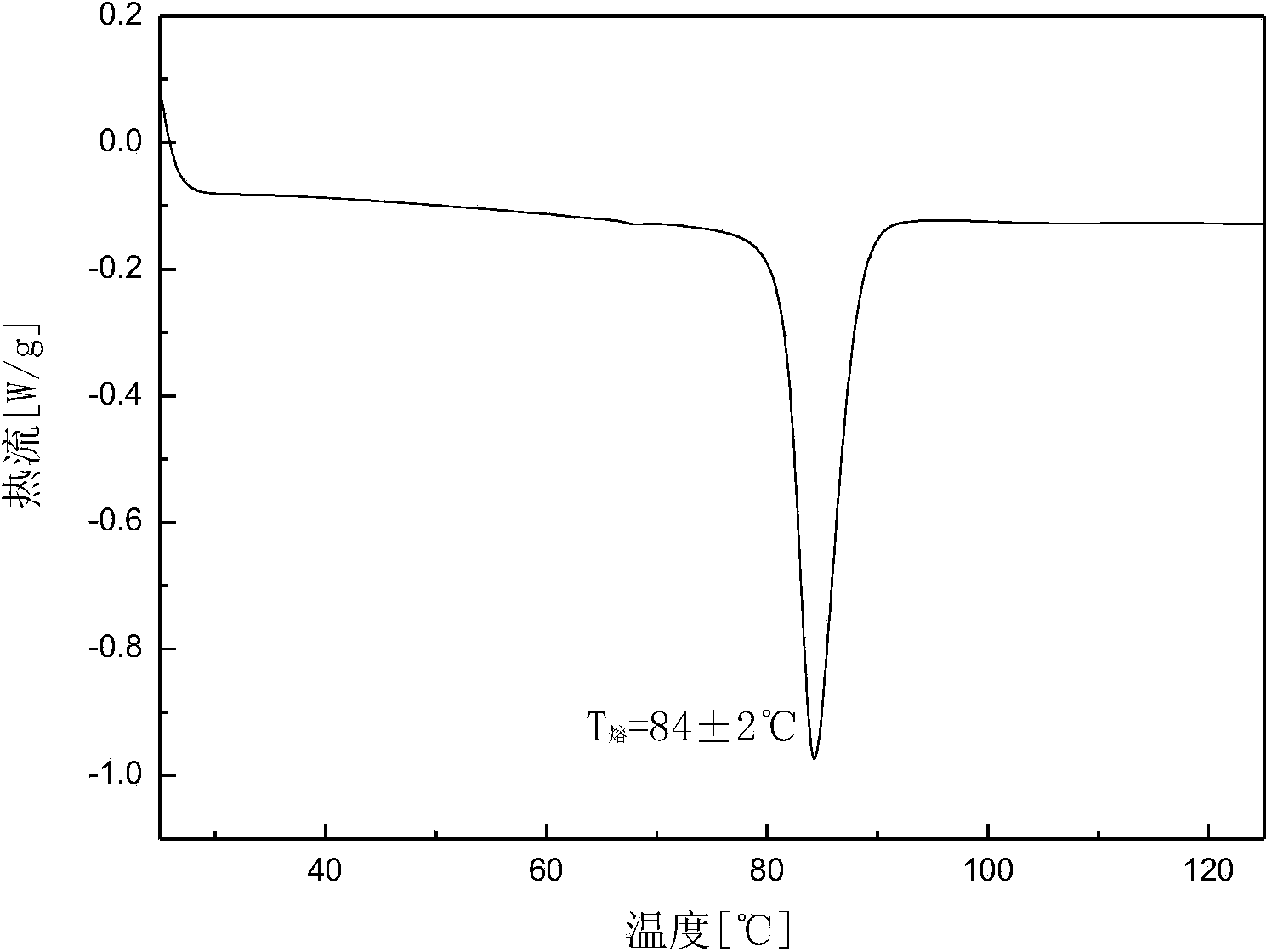
[0031] Add 10.02 g of dry canagliflozin with a purity of 92.0% into 100 mL of isoamyl alcohol to form a suspension, heat the suspension to 48 ° C under stirring, and dissolve all the solids, and add to the solution at a speed of 0.3 mL / min 300mL of n-butane was added dropwise to obtain a suspension; then starting from 48°C, 80mL of solvent was evaporated within 5 hours; the suspension was filtered to obtain a wet crystal product, which was dried at 20°C under normal pressure for 10 hours to constant Weight, to obtain B crystal form canagliflozin product. The X-ray powder diffraction pattern of the product is as follows: figure 1 As shown, it has characteristic peaks at diffraction angles 2θ=3.42, 6.64, 12.64, 13.27, 15.30, 15.65, 16.59, 19.45, 19.88, and 23.79 degrees. The DSC analysis diagram is as follows figure 2 As shown, there is an endothermic peak at 84.2°C, and its micrograph is shown in image 3 shown.
[0032] The prepared crystal form B canagliflozin product has...
Embodiment 2
[0034] Add 21.98 g of dry canagliflozin with a purity of 93.0% into 100 mL of n-propanol to form a suspension, heat the suspension to 55 °C under stirring, and dissolve all the solids, and add to the solution at a speed of 8.5 mL / min A mixed eluent of 550 mL of n-heptane and 300 mL of cyclohexane was added dropwise to obtain a suspension, and then from 55° C., 380 mL of solvent was evaporated within 10 hours; the obtained suspension was filtered to obtain a wet crystal product, and It was dried at 45° C. under normal pressure for 7 hours to constant weight to obtain crystal form B canagliflozin product. The X-ray powder diffraction pattern of the product has characteristic peaks at diffraction angles 2θ=3.41, 6.69, 12.67, 13.25, 15.30, 15.66, 16.58, 19.46, 19.88, 23.78 degrees, and DSC has an endothermic peak at 84.8°C.
[0035]The prepared crystal form B canagliflozin product has good stability, with a purity of 99.1% and a yield of 93.6%. After the product is stored under no...
Embodiment 3
[0037] Add 35.55 g of dry canagliflozin with a purity of 95.0% into 20 mL of sec-butanol and 80 mL of n-propanol to form a suspension, and heat the suspension to 60 °C under stirring to dissolve all the solids. Add 500mL of n-hexane dropwise to the solution at a high speed to obtain a suspension; then start from 60°C and evaporate 360mL of solvent within 8 hours; Dry for 6 hours to constant weight to obtain crystal form B canagliflozin product. The X-ray powder diffraction pattern of the product has characteristic peaks at diffraction angles 2θ=3.40, 6.69, 12.64, 13.26, 15.30, 15.65, 16.57, 19.43, 19.86, and 23.79 degrees, and DSC has an endothermic peak at 83.9°C.
[0038] The prepared crystal form B canagliflozin product has good stability, with a purity of 99.2% and a yield of 94.5%. After the product is stored under normal temperature and dry conditions for 100 days, the product has no change in purity, color and shape. The product is easy to crush and easy to add to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com