C crystal form of canagliflozin and crystal preparation method of canagliflozin
A technology of canagliflozin and crystal form, which is applied in the field of fine chemical industry and application, can solve the problems of many steps in the crystallization method, and achieve the effect of low cost, high solubility, and easy commercial industrialization scale implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
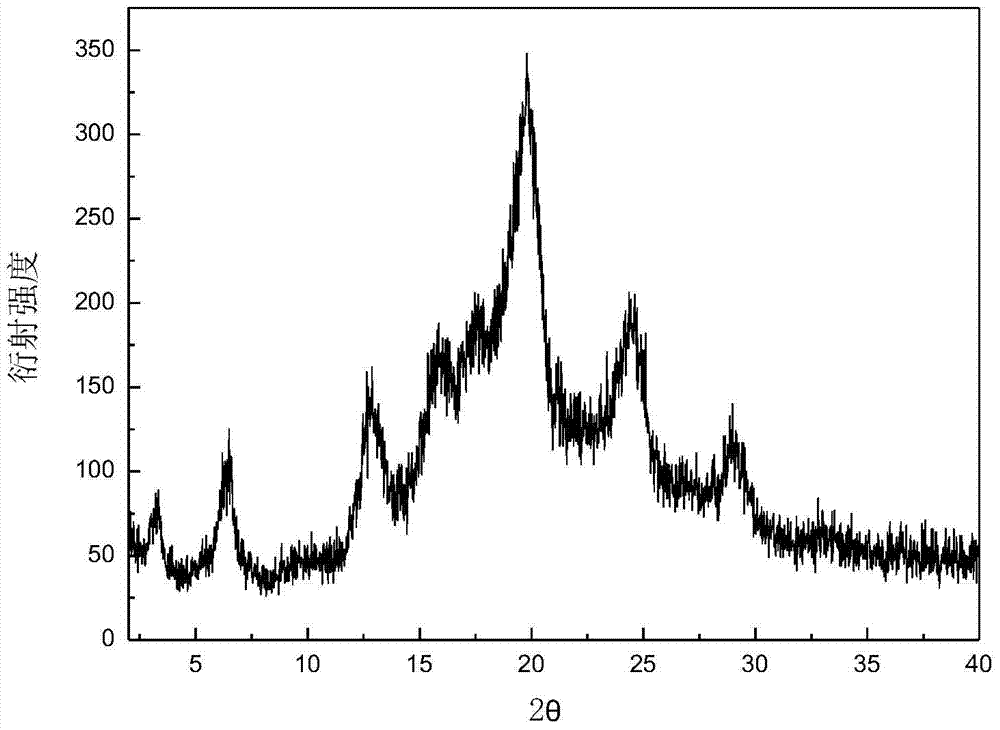
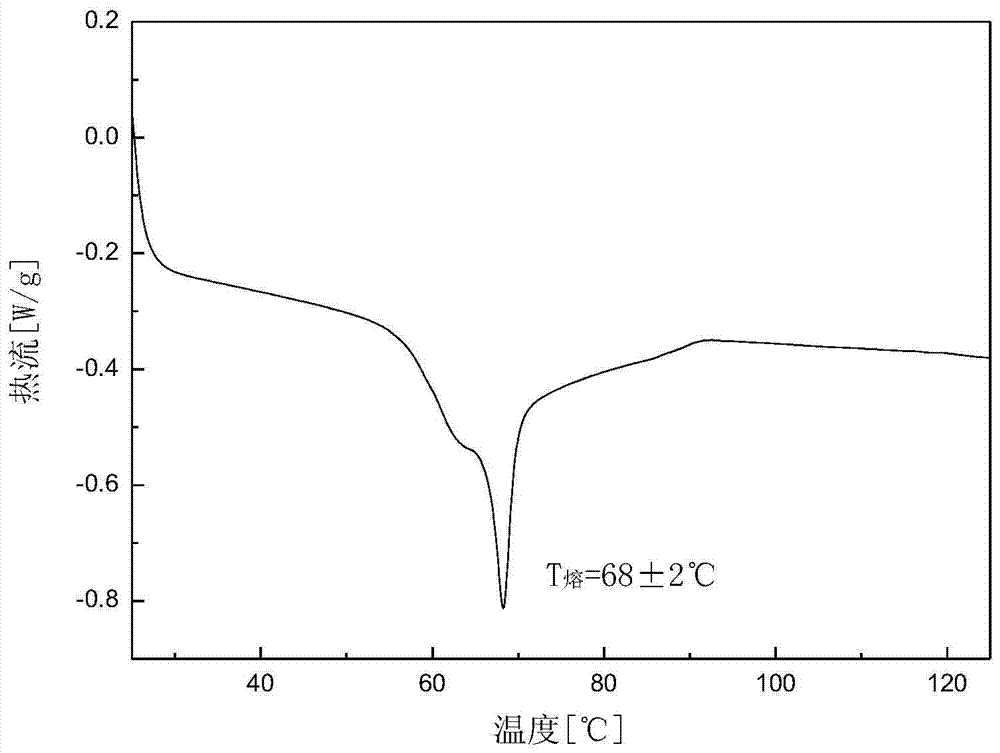
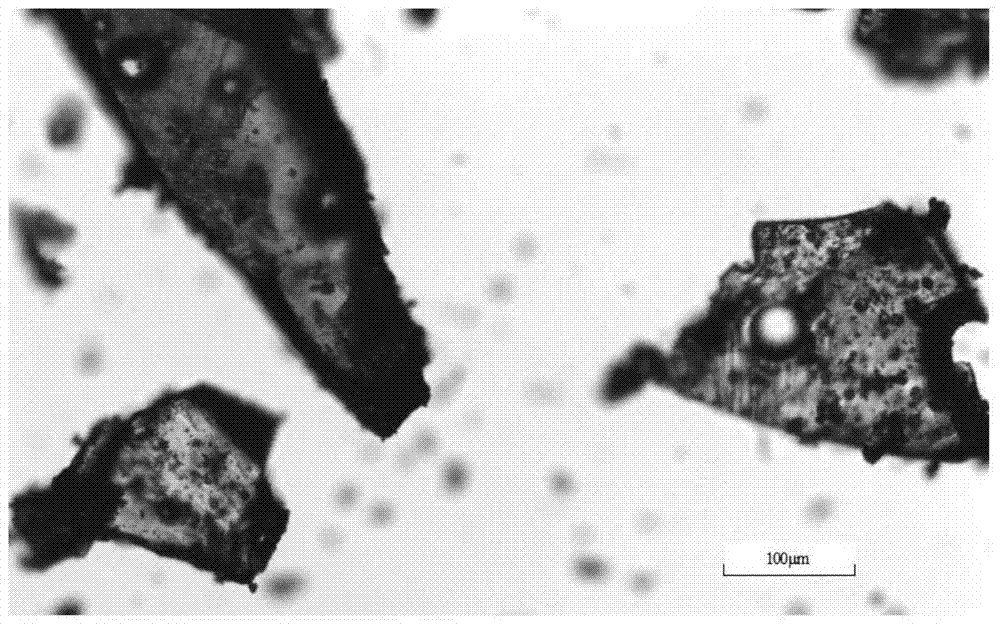
[0031] Add 5.08 g of dry canagliflozin solids with a purity of 92.0% into 100 mL of isopropanol to form a suspension, heat the suspension to 35 °C under stirring, and dissolve all the solids. Add 200mL of n-hexane dropwise to the solution, then cool down to 5°C at a rate of 0.05°C / min to obtain a suspension; filter the resulting suspension and dry the obtained wet product at 30°C and a vacuum of 0.07MPa for 10 hours The crystal form C product of canagliflozin was obtained. The X-ray powder diffraction pattern of the product is as follows: figure 1 As shown, it has characteristic peaks at diffraction angles 2θ=3.42, 6.55, 12.74, 15.89, 19.85, 24.37, 24.80, and 29.13 degrees; the DSC analysis diagram is as follows figure 2 As shown, it has a characteristic endothermic peak at 68.3 °C. Microscopic pictures of the crystal shape as image 3 shown.
[0032] The prepared crystal form C canagliflozin product has good stability, with a purity of 99.3% and a yield of 89.9%. After t...
Embodiment 2
[0034] Add 13.28 g of dried canagliflozin solids with a purity of 94.0% into 100 mL of absolute ethanol to form a suspension, heat the suspension to 55 °C under stirring, and dissolve all the solids. Add 250mL of n-octane and 200mL of mixed eluent of n-butyl ether dropwise into the solution, then cool down to -5°C at a rate of 1.5°C / min to obtain a suspension; filter the resulting suspension and filter the resulting wet product Drying for 7 hours at 40°C and a vacuum of 0.1 MPa to obtain the crystal form C product of canagliflozin. The X-ray powder diffraction pattern of the product has characteristic peaks at diffraction angles 2θ=3.43, 6.56, 12.74, 15.88, 19.85, 24.38, 24.82, and 29.15 degrees, and the DSC analysis diagram has characteristic endothermic peaks at 69.1°C.
[0035]The prepared crystal form C canagliflozin product has good stability, with a purity of 99.1% and a yield of 91.1%. After the product was stored under normal temperature and dry conditions for 100 days...
Embodiment 3
[0037] Add 22.03 g of dried canagliflozin solid with a purity of 93.0% into 30 mL of acetone and neutralize 70 mL of n-butanol to form a suspension, and heat the suspension to 65 ° C while stirring to dissolve all the solids. Add 600mL of cyclohexane dropwise to the solution at a speed of 0.3°C / min to -20°C to obtain a suspension; filter the resulting suspension and store the wet product at 35°C and a vacuum of 0.09MPa Drying at lower temperature for 8 hours to obtain the crystal form C product of canagliflozin. The X-ray powder diffraction pattern of the product has characteristic peaks at diffraction angles 2θ=3.43, 6.55, 12.74, 15.88, 19.86, 24.38, 24.83, and 29.14 degrees, and the DSC analysis diagram has characteristic endothermic peaks at 68.8°C.
[0038] The prepared crystal form C canagliflozin product has good stability, with a purity of 99.2% and a yield of 92.3%. After the product was stored under normal temperature and dry conditions for 100 days, the product purit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com