2-(4'-methoxy phenoxy)-4-nitro methanesulfonanilide crystal form and preparation method thereof
A technique for methosulide crystal and crystallization, applied in the field of methosulide crystal form and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation Mesosulide
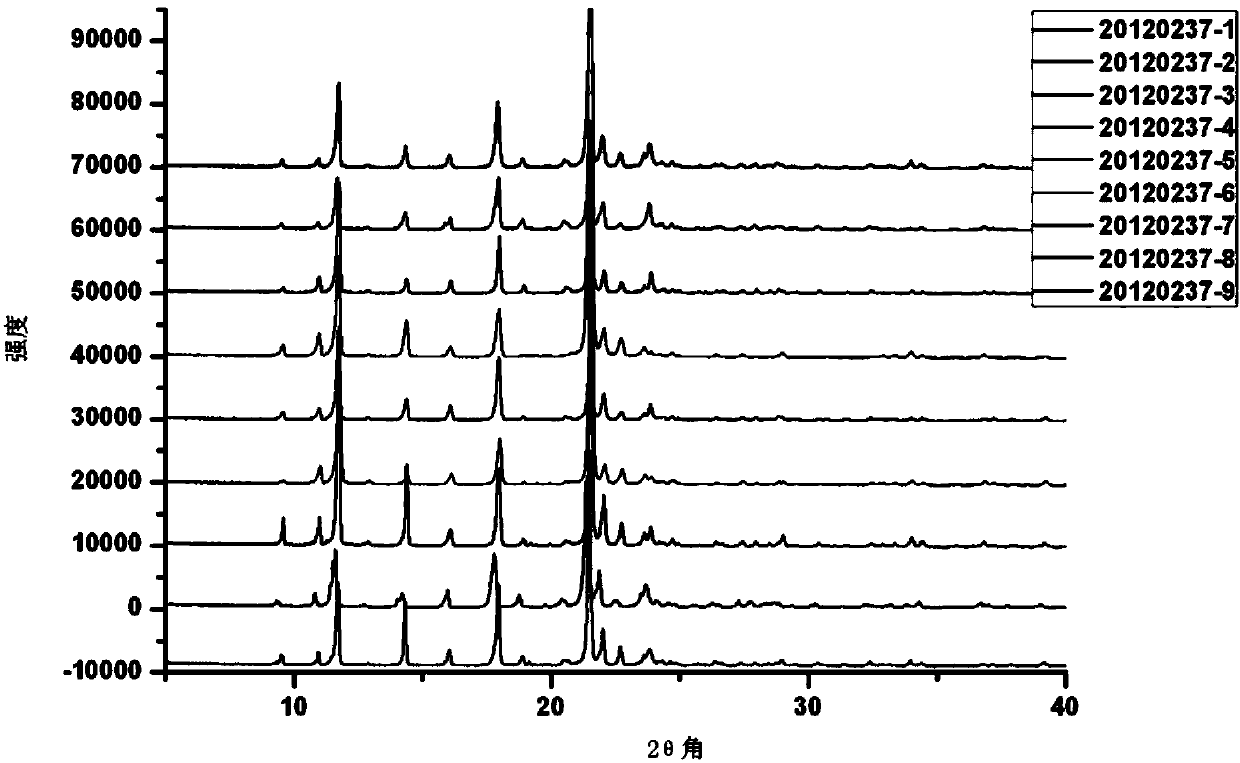
[0045] Add 2-(4'-methoxyphenoxy)-methanesulfonanilide and acetic acid into the reaction kettle, stir, after completely dissolving, add nitric acid, after the reaction is completed, cool at room temperature, and collect the precipitated yellow Solid, washed with water until neutral, dried to obtain crude Mesosulide. The crude product was recrystallized with ethanol, heated to reflux and filtered, then crystallized naturally, the solid was collected by filtration, and dried in vacuo to obtain light yellow crystals or crystalline powder, i.e. mesosulide, with a yield of 60.4%. Qualified samples of mesosulide produced in three consecutive batches of pilot scale scale-up,
[0046] Batch number: 20120202 (analysis number W20120237-7),
[0047] 20120314 (analysis number W20120237-8),
[0048] 20120316 (Assay No. W20120237-9).
Embodiment 2
[0050] Mesosulide batch number: 20120509-1 (analysis number W20120237-1): Take 1g of crude mesosulide in a 50ml single-mouth bottle, put it into a magnet, add 20ml of ethanol, heat and reflux for 2h to completely dissolve, cool and crystallize, Filter, dry, and send for inspection.
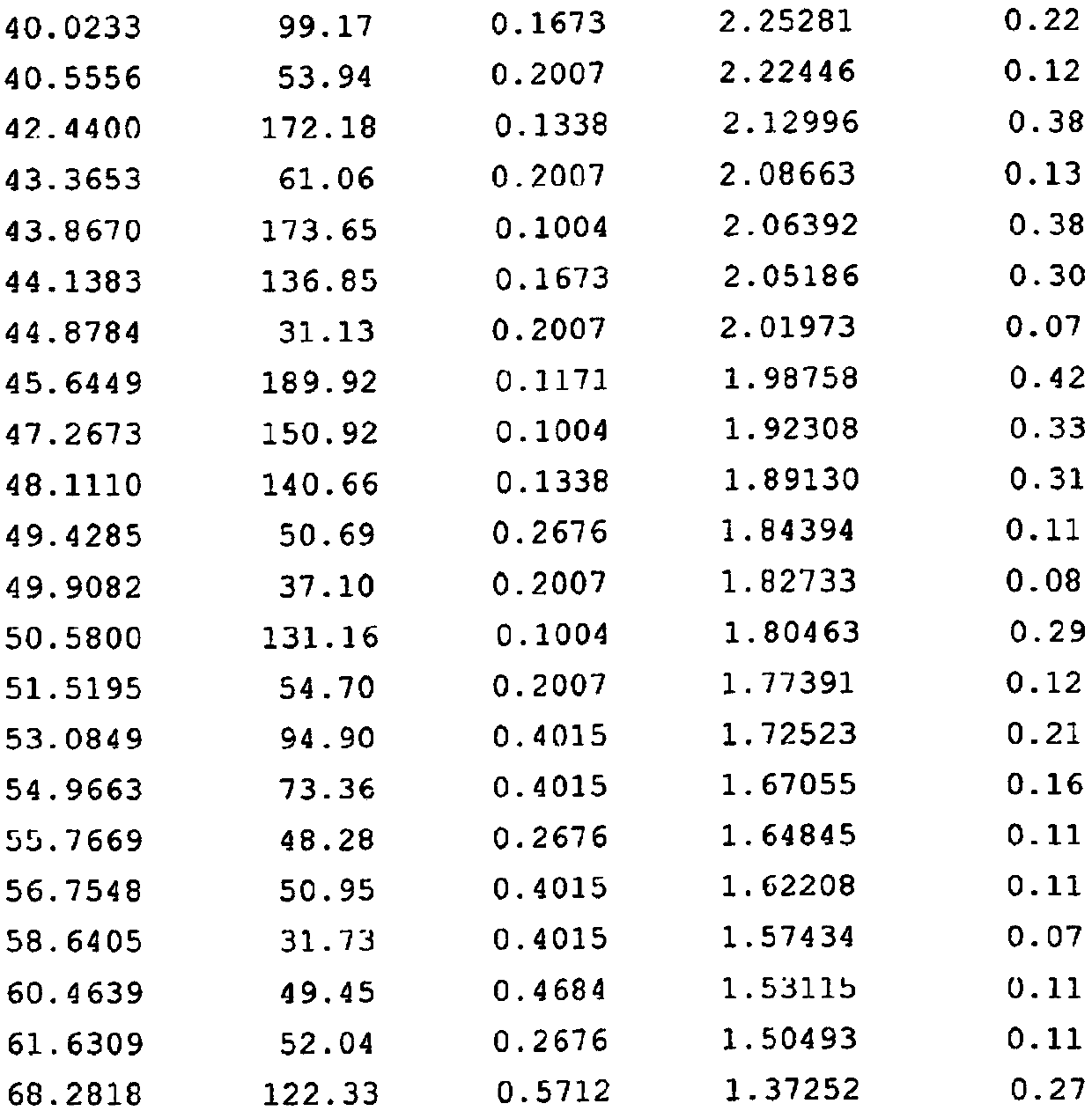
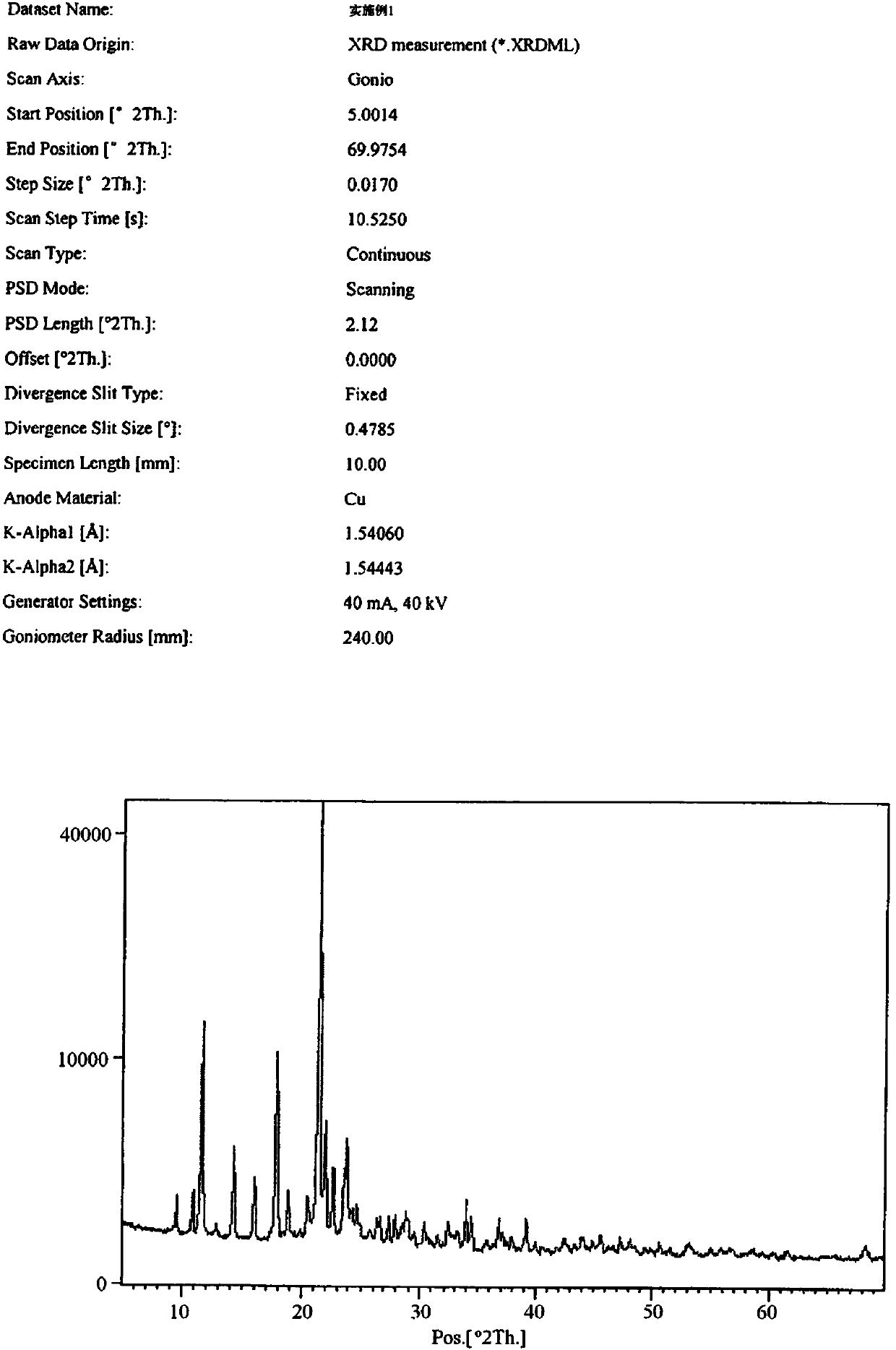
[0051] figure 1 It is the X-ray diffraction experimental conditions and diffraction pattern of the above-mentioned mesosulide crystal with batch number 20120509-1 (analysis number W20120237-1);
[0052] figure 2 yes figure 1 The relevant data of the X-ray diffraction peak in;
[0053] Figure 4 It is the DSC chart of the above-mentioned mesosulide crystal with batch number 20120509-1 (analysis number W20120237-1).
Embodiment 3
[0055] Methosulide batch number: 20120509-3 (analysis number W20120237-2): Take 1g of crude mesosulide in a 50ml single-mouth bottle, put it into a magnet, add 20ml of methanol, heat and reflux for 2h to completely dissolve, cool and crystallize, Filter, rinse with ethanol, rinse with ethanol, dry, and send for inspection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com