Canagliflozin of crystal form A, and crystallization preparation method thereof
A crystal form and crystal technology, which is applied to the new crystal form of canagliflozin and its crystallization preparation field, can solve the problems of many steps in the crystallization method, and achieve the effects of low cost, short time consumption and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
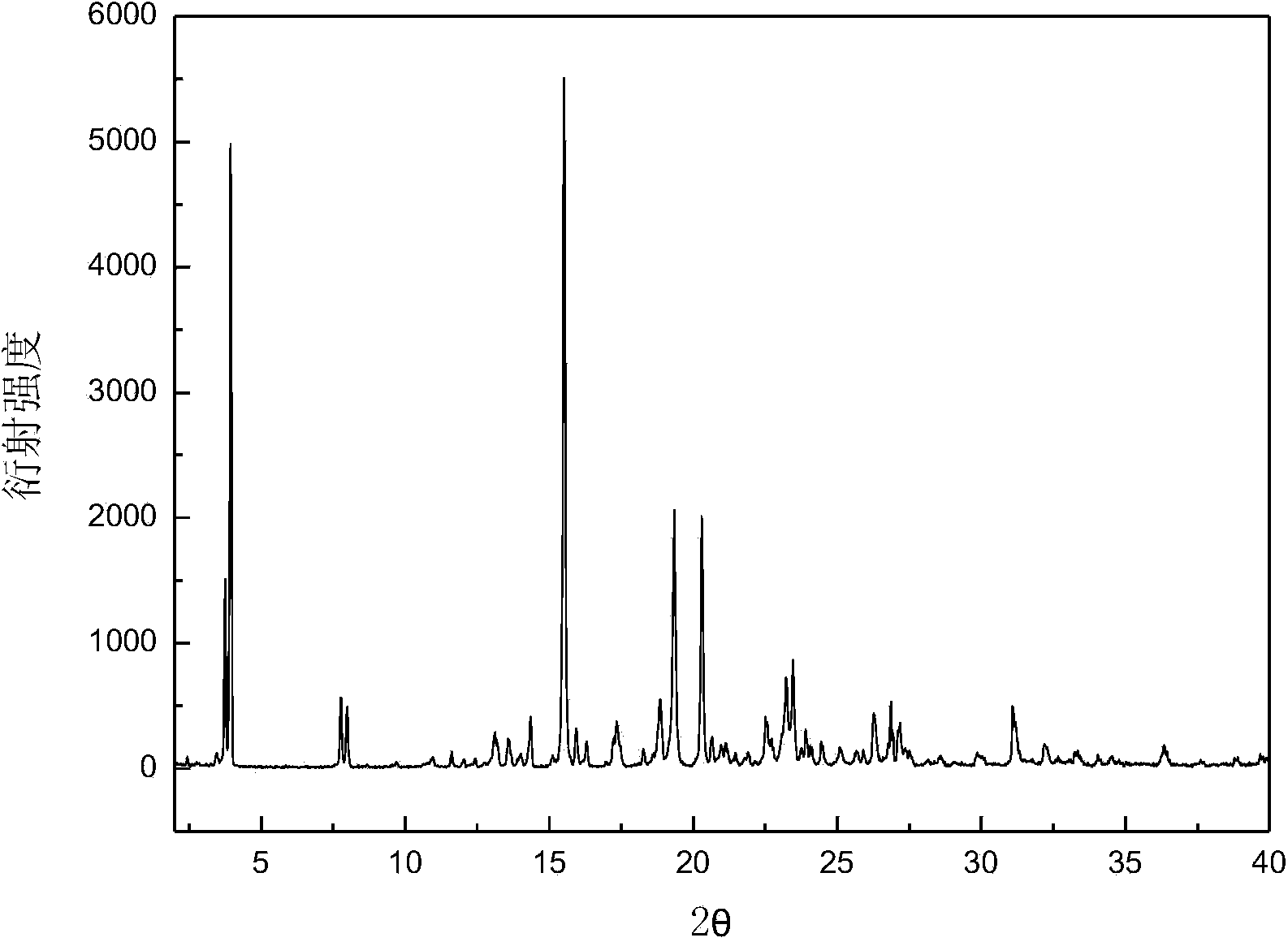
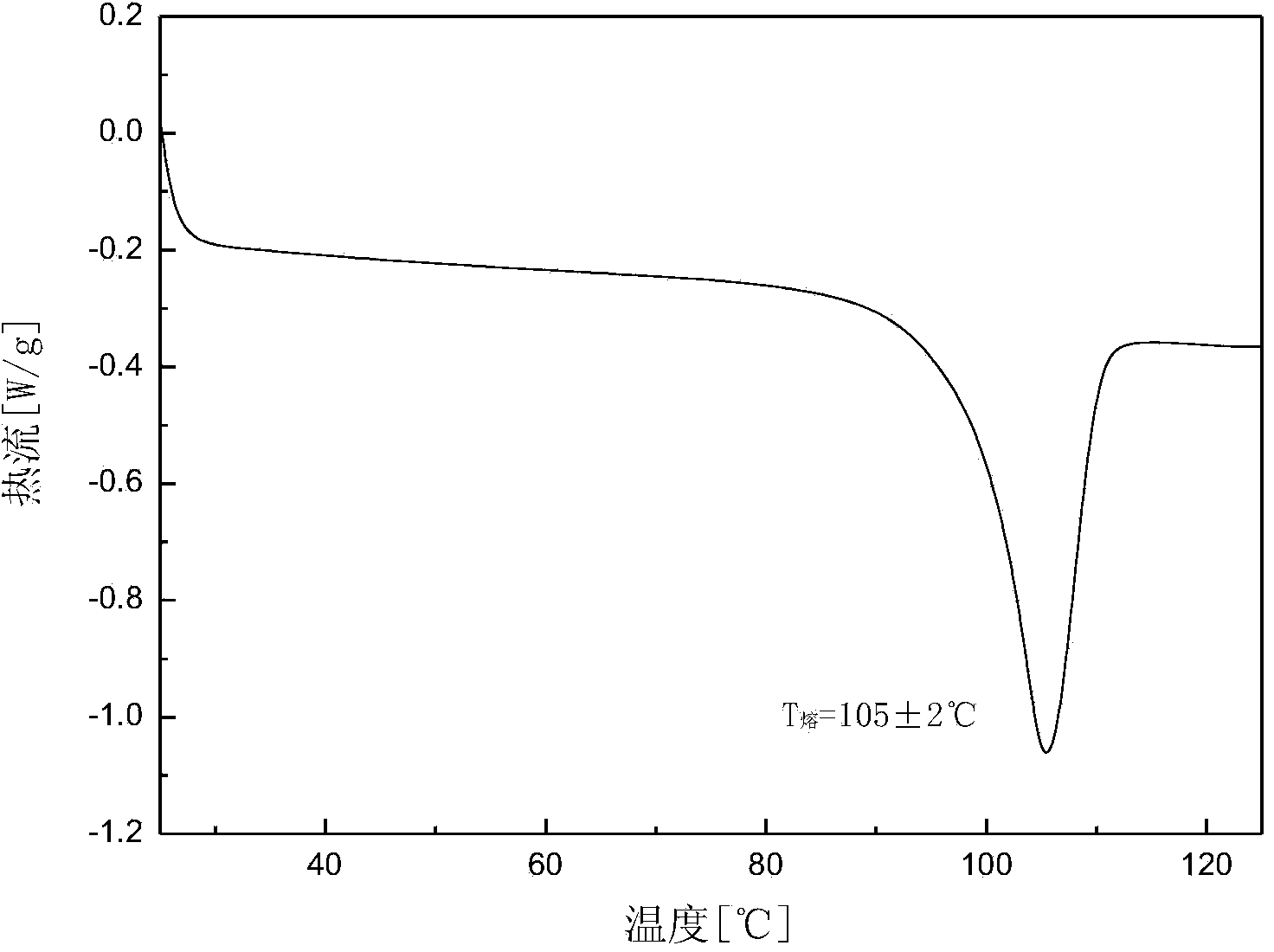
[0029] Add 5.1 g of dry canagliflozin solid with a purity of 92.0% into 100 mL of n-propanol to form a suspension, and heat the suspension to 15 °C under stirring to dissolve all the solids; Add 300 mL of cyclohexane dropwise to the solution to obtain a suspension, vacuum filter the crystal slurry, and dry the product at 30°C under normal pressure for 8 hours to constant weight to obtain the crystal form A canagliflozin product. The X-ray powder diffraction pattern of the product is as follows: figure 1 As shown, it has characteristic peaks at diffraction angles 2θ=3.71, 3.94, 7.77, 7.92, 11.53, 13.16, 13.58, 14.30, 15.56, 17.32, 18.81, 19.36, 20.31, 22.50, 22.79, 23.24, 23.48 degrees, DSC analysis Figure such as figure 2 , which has a characteristic endothermic peak at 105.4°C. Microscopic pictures of the crystal shape as image 3 shown.
[0030] The prepared crystal form A canagliflozin product has good stability, with a purity of 99.3% and a yield of 88.3%. After the p...
Embodiment 2
[0032] Add 14.3 g of dry canagliflozin solids with a purity of 96.0% into 100 mL of isopropanol to form a suspension, and heat the suspension to 25 °C under stirring to dissolve all the solids; Add 400mL of n-hexane and 100mL of water as a mixed eluent dropwise to obtain a suspension, vacuum filter the crystal slurry, and dry the product at 45°C under normal pressure for 7 hours to constant weight to obtain the A crystal form net. The X-ray powder diffraction pattern of the product has characteristics at diffraction angles 2θ=3.73, 3.96, 7.78, 7.94, 11.53, 13.15, 13.57, 14.31, 15.58, 17.34, 18.82, 19.35, 20.32, 22.52, 22.79, 23.25, 23.48 degrees Peak, DSC analysis chart has a characteristic endothermic peak at 105.56°C.
[0033] The prepared crystal form A canagliflozin product has good stability, with a purity of 99.1% and a yield of 89.6%. After the product was stored under normal temperature and dry conditions for 100 days, the product purity, color and shape did not chang...
Embodiment 3
[0035] Add 28.5 g of dried canagliflozin solids with a purity of 94.0% into 40 mL of ethanol and 60 mL of n-pentanol to form a suspension, and heat the suspension to 38 °C under stirring to dissolve all the solids; Add 650 mL of petroleum ether dropwise to the solution at a high speed to obtain a suspension, vacuum filter the crystal slurry, and dry the product at 45°C under normal pressure for 6 hours to constant weight to obtain crystal form A of canagliflozin. The X-ray powder diffraction pattern of the product has characteristics at diffraction angles 2θ=3.74, 3.96, 7.77, 7.95, 11.55, 13.17, 13.58, 14.32, 15.59, 17.36, 18.82, 19.36, 20.33, 22.53, 22.78, 23.25, 23.48 degrees Peak, DSC analysis chart has a characteristic endothermic peak at 106.72°C.
[0036] The prepared crystal form A canagliflozin product has good stability, with a purity of 99.2% and a yield of 90.5%. After the product was stored under normal temperature and dry conditions for 100 days, the product purit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com