Compound for the treatment of gastrointestinal disease
A technology of sesquihydrate and pantoprazole sodium, which is applied in the field of compounds for the treatment of gastrointestinal diseases, can solve the problems of crystal form description, poor chemical stability and wet stability, instability, etc., and achieve good stability, containing The effect of stable water volume and low hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
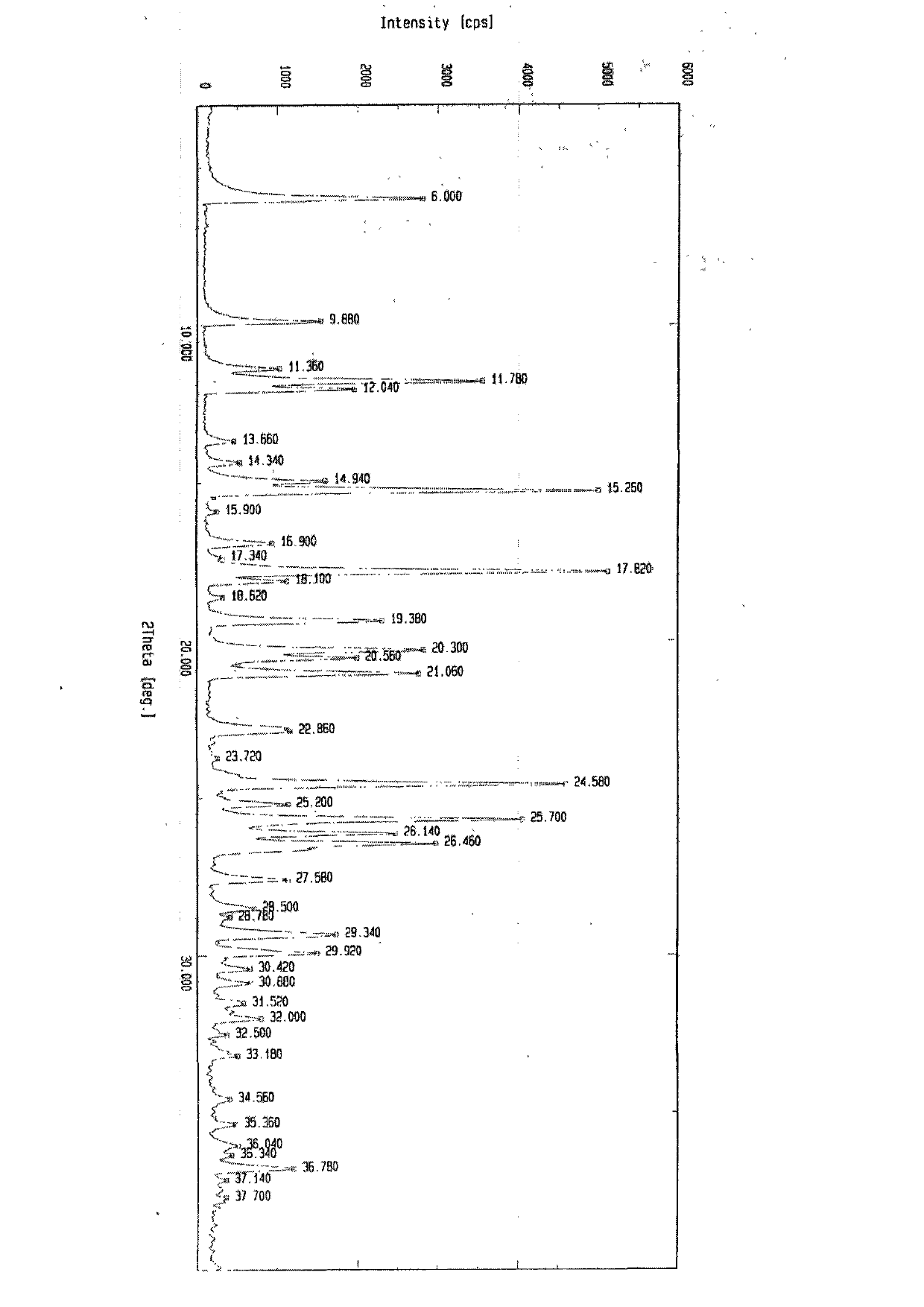
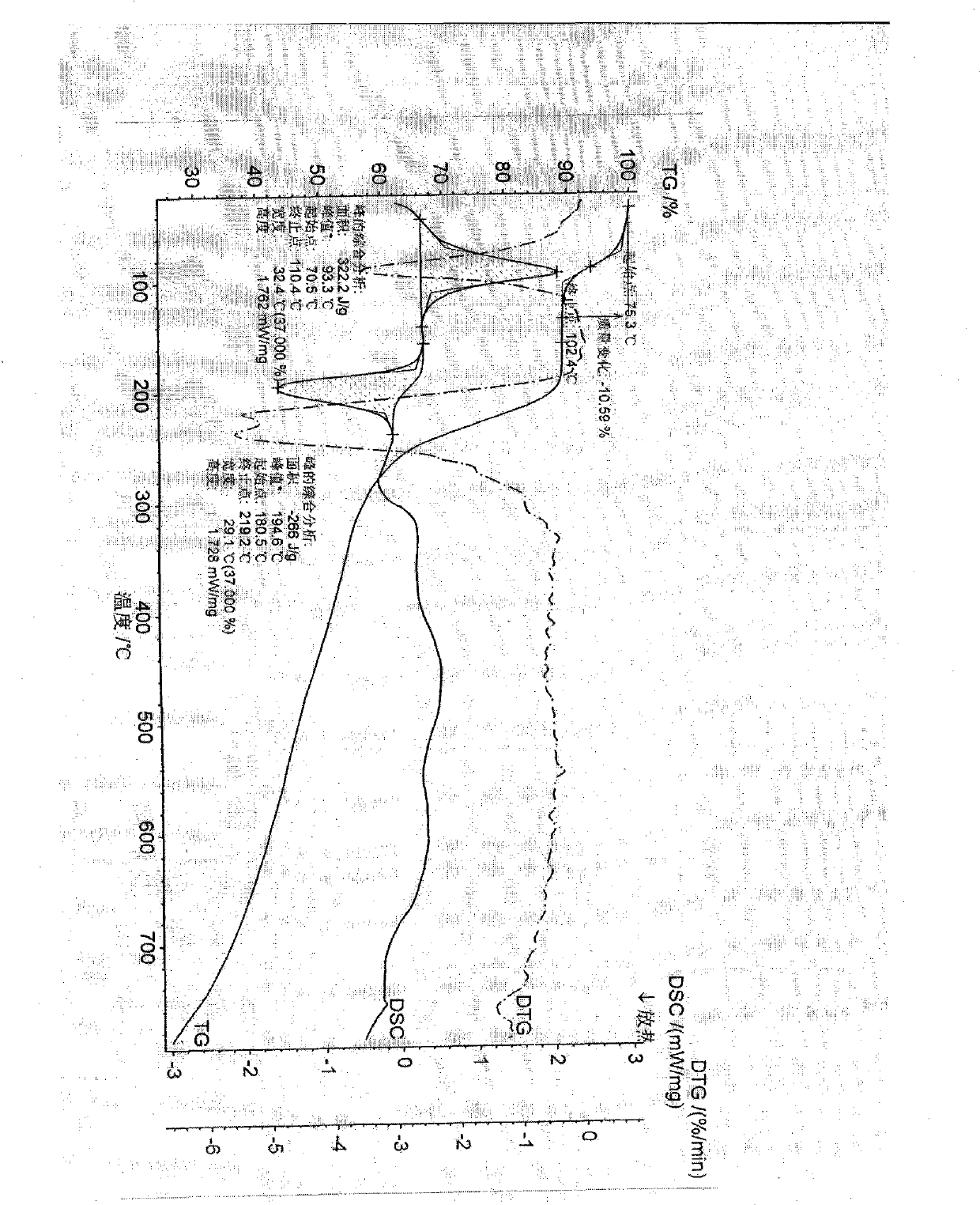
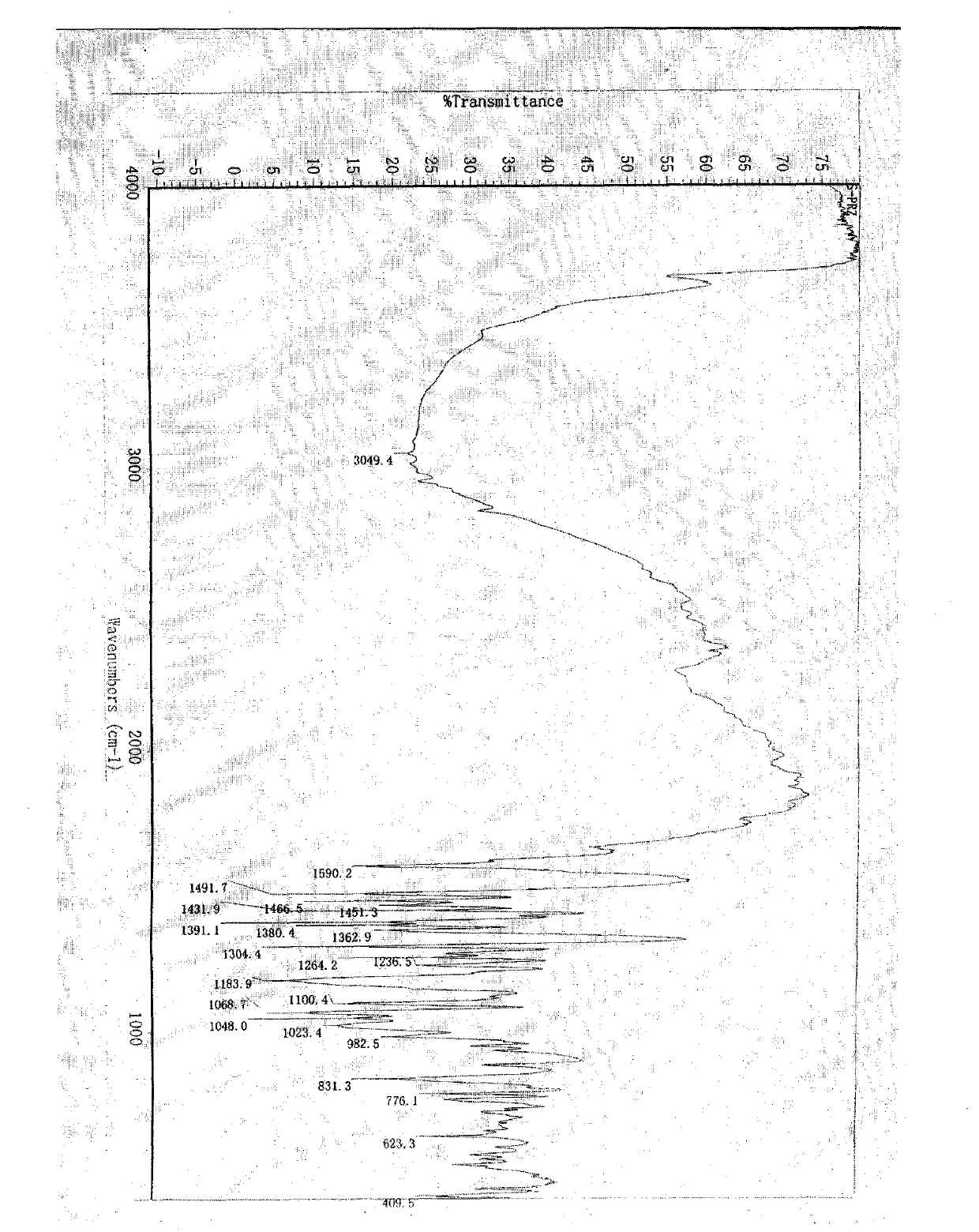
Image
Examples
Embodiment 1
[0031] S-pantoprazole 17g, 340ml aqueous solution that is dissolved with 2.2gNaOH is put into the reaction flask, stir at room temperature until the material is completely dissolved, filter the feed liquid, and distill under reduced pressure at a temperature of 35°C. Stop the distillation when there is solid precipitation in the feed liquid, and place it in a refrigerator at 0° C. for crystallization for 48 hours. Filter the feed liquid, wash the filter cake with a small amount of cooled water, and dry at 50° C. under normal pressure for 6 hours to obtain 17 g of S-pantoprazole sodium di-sesquihydrate solid, with a yield of 85%. The moisture content determined by the Fischer method was 10.1%, and the related substances determined by HPLC were 99.8%.
Embodiment 2
[0033]17 g of S-pantoprazole, 34 ml of aqueous solution containing 5.2 g of NaOH, and 34 ml of methanol were put into a reaction flask, stirred at a temperature of 5° C. until the material was completely dissolved, filtered, and distilled under reduced pressure at a temperature of 40° C. Stop the distillation when there is solid precipitation in the feed liquid, and place it in a refrigerator at 10° C. for 24 hours for crystallization. Filter the feed liquid, wash the filter cake with a small amount of cooled water, and dry at 40° C. under normal pressure for 10 hours to obtain 18 g of S-pantoprazole sodium di-sesquihydrate solid, with a yield of 90%. The moisture content determined by the Fischer method was 10.2%, and the related substances determined by HPLC were 99.8%.
Embodiment 3
[0035] 17 g of S-pantoprazole, 85 ml of aqueous solution with 1.8 g of NaOH, and 25 ml of ethanol were put into a reaction flask, stirred at a temperature of 25° C. until the material was completely dissolved, filtered the feed liquid, and distilled under reduced pressure at a temperature of 30° C. of the feed liquid. Stop the distillation when there is solid precipitation in the feed liquid, and place it in a refrigerator at 5° C. for 8 hours for crystallization. Filter the feed liquid, wash the filter cake with a small amount of cooled water, and dry at 40° C. under normal pressure for 8 hours to obtain 18 g of S-pantoprazole sodium di-sesquihydrate solid, with a yield of 90%. The moisture content determined by Fischer's method was 10.5%, and the related substances determined by HPLC were 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com