Stable S-oxiracetam preparation for injection and preparation method of same
A technology for injection and preparation, applied in the field of medicine, can solve the problems such as loss of pharmacological activity advantage of S-oxiracetam
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
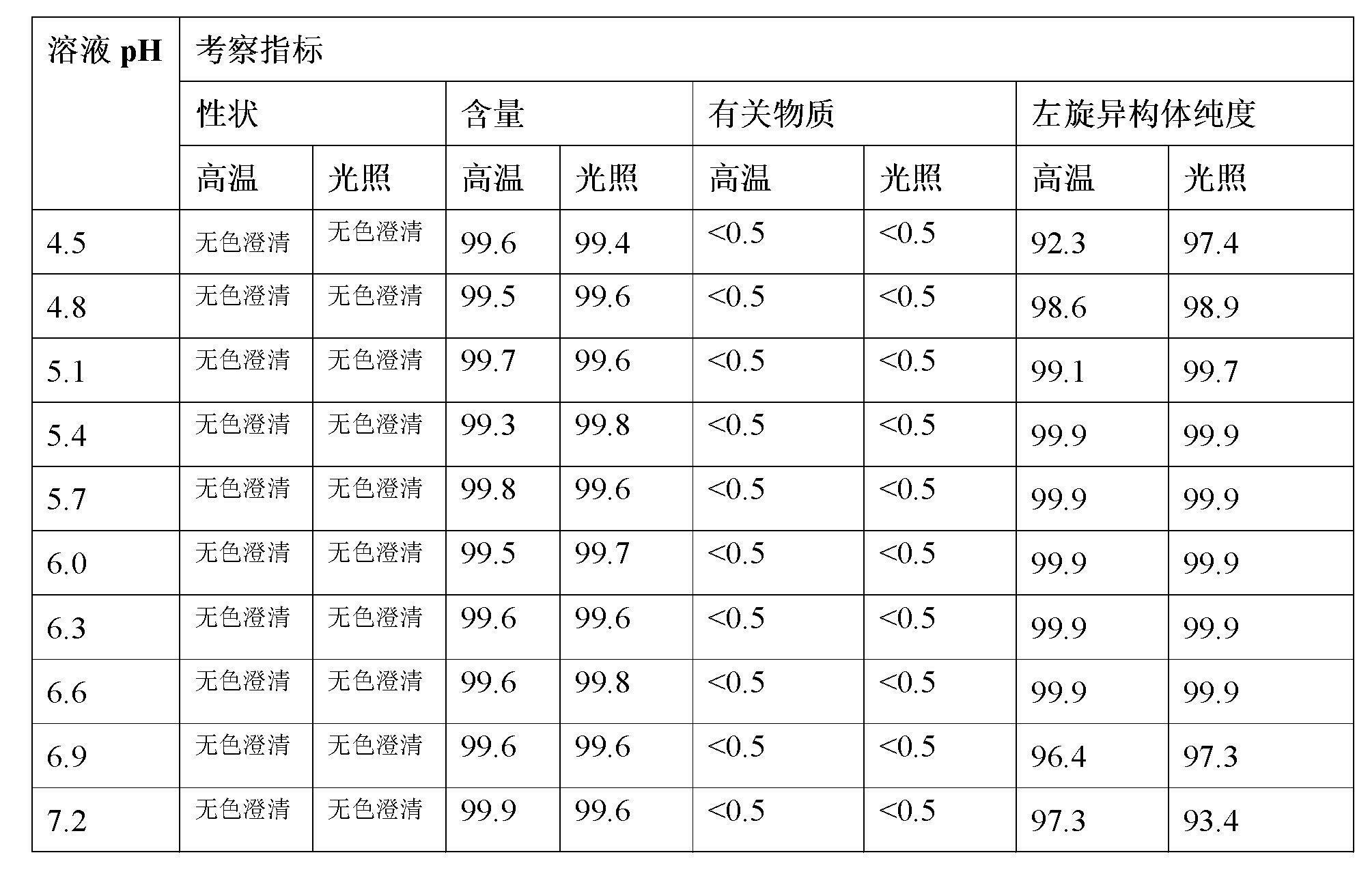
[0017] Example 1 S-oxiracetam drug solution pH screening
[0018] Take 5g of S-oxiracetam, add 100mL of water to dissolve, measure the pH to be about 1.8, add EDTA sodium calcium to dissolve in an appropriate amount, divide the solution into several equal parts, and use sodium hydroxide / hydrochloric acid to adjust the pH of the solution to 4.5, 4.8, 5.1, 5.4, 5.7, 6.0, 6.3, 6.6, 6.9, and 7.2, after being adsorbed by activated carbon, decarbonized by filtration, sealed in ampoules. The color appearance, content, related substances and isomer purity of S-oxiracetam were investigated respectively at the initial stage, under high temperature (℃) and under accelerated light conditions for 30 days. The content is based on the relative content of the initial result. The result obtained is as follows:
[0019]
[0020] The above results suggest that within the investigated pH range, the content of S-oxiracetam has no significant change, suggesting that no hydrolysis or other degr...
Embodiment 2
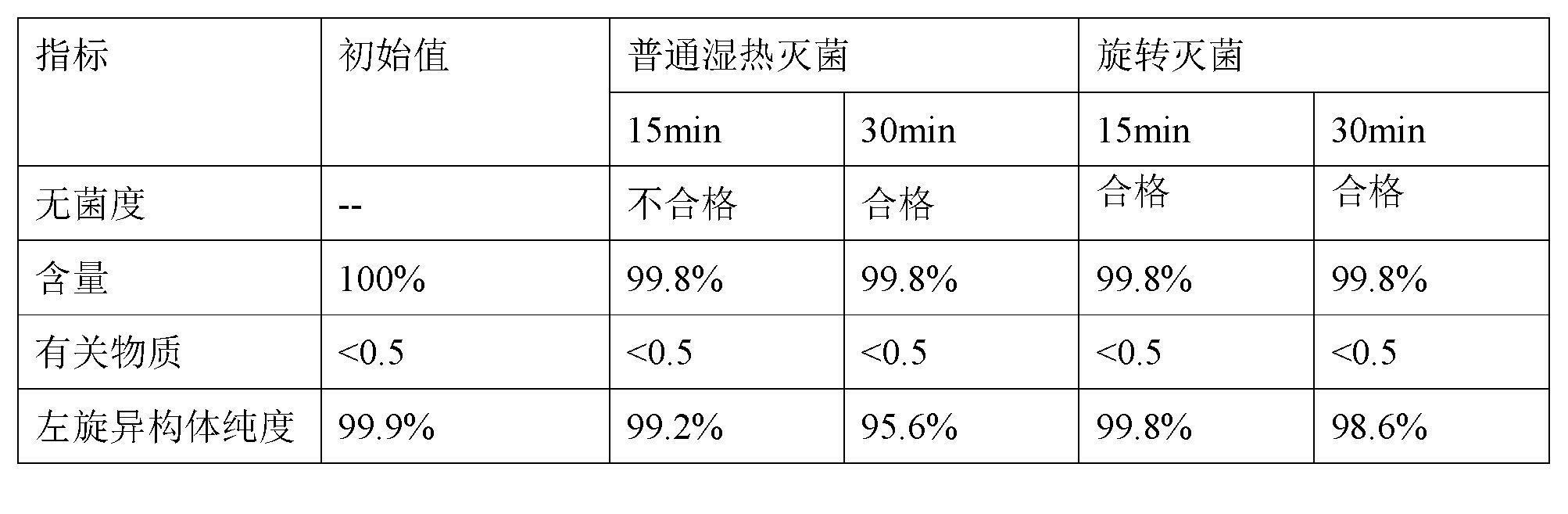
[0021] Example 2 Effect of heat exposure on racemization of S-oxiracetam
[0022] Take 100g of S-oxiracetam, add 10000mL of water to dissolve it, add an appropriate amount of EDTA sodium calcium to dissolve, and adjust the pH of the solution to 6.0 with sodium hydroxide / hydrochloric acid, absorb it with activated carbon, filter and decarbonize, and seal it in 100ml infusion inside the bottle. The sterility, content, related substances, and Isomeric purity of S-oxiracetam. The content is based on the relative content of the initial result. The result obtained is as follows:
[0023]
[0024] The above obtained results suggest that: due to the rapid and uniform temperature rise of the material in the rotary sterilization process, it can achieve the purpose of sterilization in a short time (within 15 minutes), and at this time the effect on the racemization of S-oxiracetam The impact is minimal, therefore, during the production of injections, the method of rotary steriliza...
Embodiment 3
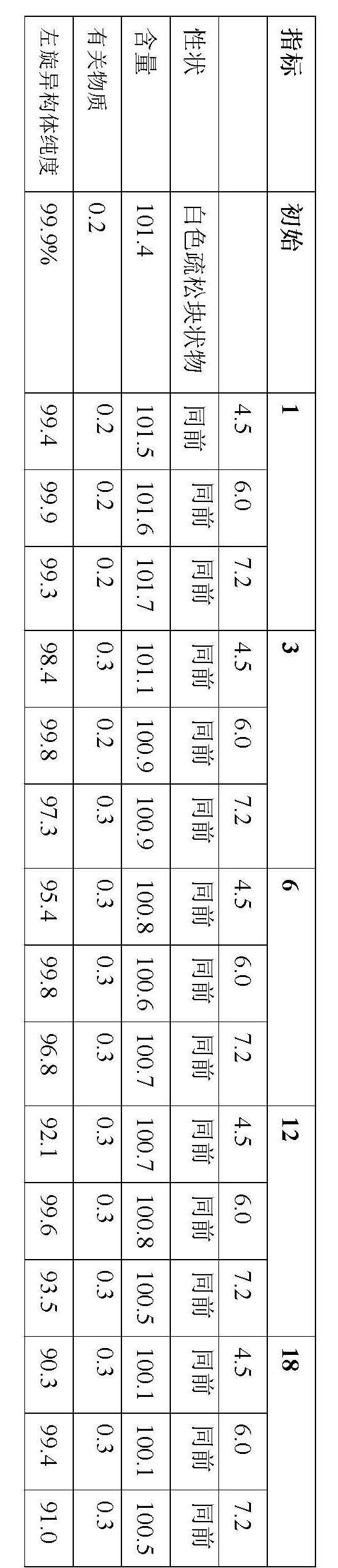
[0025] Example 3 Effect of pH of S-oxiracetam drug preparation solution on long-term stability of drug preparation (purity of L-isomer)
[0026] Take 50g of S-oxiracetam, add 250mL of water to dissolve it, add an appropriate amount of sodium calcium EDTA to dissolve it, and adjust the pH of the solution to 4.5, 6.0 and 7.2 with sodium hydroxide / hydrochloric acid, after adsorption by activated carbon and decarbonization by filtration, One part was filled in ampoules and freeze-dried; the other part was sealed in ampoules, rotatable (121°C, 15 minutes), and stored at room temperature for 1, 3, 6, 12 and 18 months. Oxiracetam appearance (powder or solution), content, related substances, isomer purity of S-oxiracetam. The content is measured in absolute content. The results obtained are shown below.
[0027] Long-term stability of S-oxiracetam lyophilized preparation
[0028]
[0029] Long-term stability of S-oxiracetam injection
[0030]
[0031] As shown in the results...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com