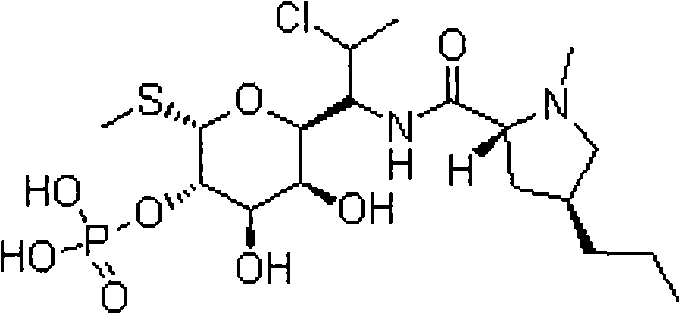
Clindamycin phosphate freeze-dried powder needle and preparation thereof
A technology of clindamycin phosphate and freeze-dried powder injection, which is applied in freeze-dried transportation, powder transportation, pharmaceutical formulations, etc., can solve the problems of cumbersome steps and increased substances, and achieve simple formula, few side effects, and full appearance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
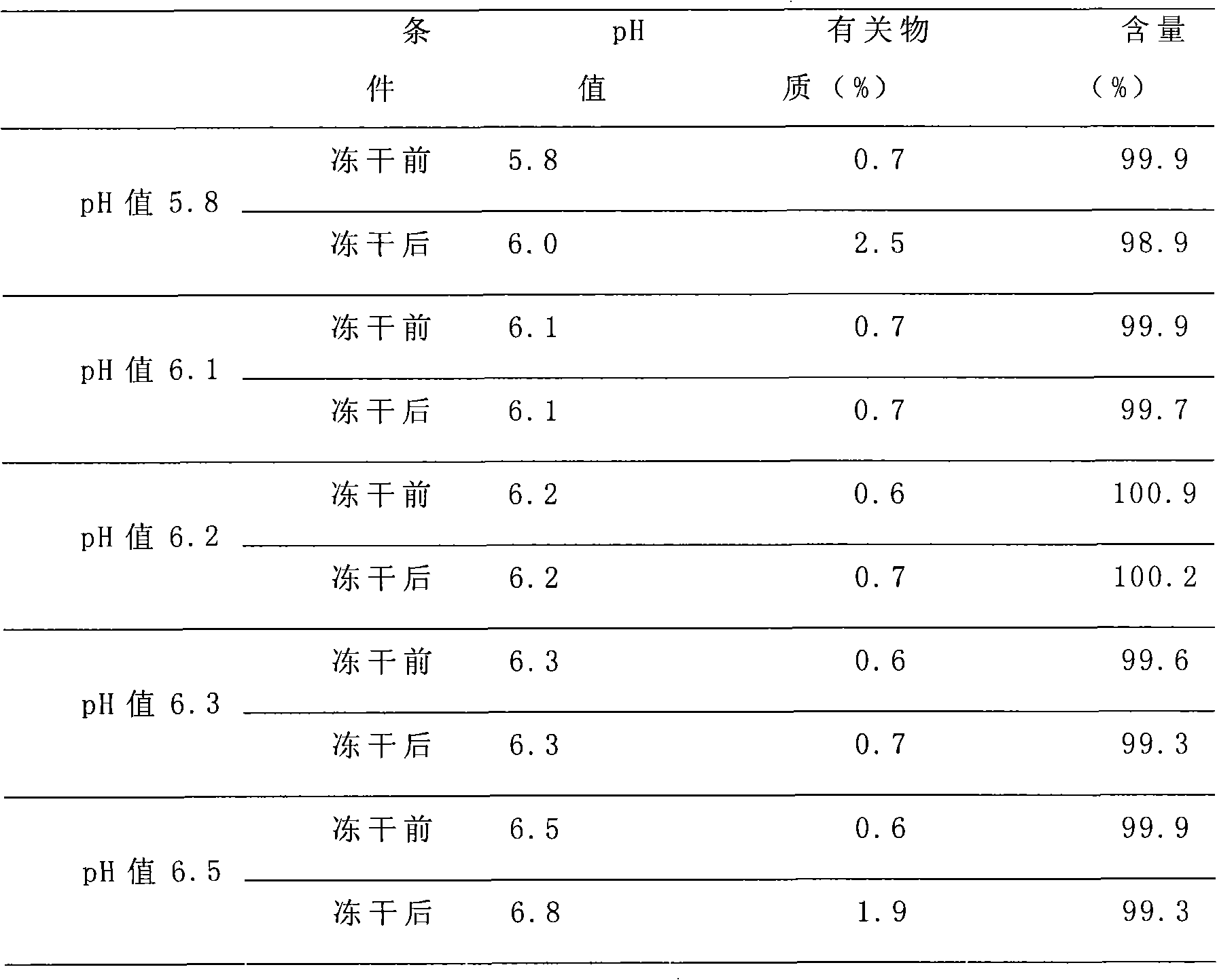
[0054] Under the condition of air cleanliness level 100,000 and local level 100, add 370.25 clindamycin phosphate (equivalent to 300g clindamycin) into 1200ml water for injection. Slowly add 280ml of 2mol / l sodium hydroxide solution while stirring to make it completely dissolved, so that the pH of the solution is between 6.10 and 6.30, add 0.03% g / mL of medicinal activated carbon, stir and adsorb for 30 minutes, Remove carbon by filter membrane, add water for injection to the filtrate to 2000mL, stir fully for 20 minutes to mix the solution evenly, and carry out terminal filtration sterilization on the prepared solution through a 0.22μm microporous filter membrane to obtain the filtrate; put the filtrate into 1000 bottles In the injection bottle, put the injection bottle containing the filtrate into the material tray and put it on the partition in the freeze-drying box in time, and pre-freeze: the temperature is -45 ° C, and the time is 4 hours; after the drug is frozen, the co...
Embodiment 2
[0056] Under the condition of air cleanliness level 100000 and local level 100, add 740.5 clindamycin phosphate (equivalent to 600g clindamycin) into 2400ml water for injection, the water for injection must be cooled to below 20 ℃ in advance, Slowly add 560ml of 2mol / l sodium hydroxide solution while stirring to make it completely dissolved, so that the pH of the solution is between 6.10 and 6.30, add 0.03% g / mL of medicinal activated carbon, stir and adsorb for 30 minutes, Filter membrane to remove carbon, add water for injection to the filtrate to 4000mL, stir fully for 20 minutes to mix the solution evenly, and filter the prepared solution through a 0.22μm microporous membrane to obtain the filtrate; put the filtrate into the injection bottle In the process, put the injection bottle containing the filtrate into the material tray and put it on the partition in the freeze-drying box in time, and pre-freeze: the temperature is -45°C, and the time is 4 hours; after the drug is f...
Embodiment 3
[0058] Under the condition of air cleanliness level 100,000 and local level 100, add 300 grams of lindamycin phosphate to 1200 ml of water for injection. The water for injection must have been cooled to below 20°C in advance, and slowly add 2 mol / l of hydroxide while stirring. Sodium solution 250ml, make it dissolve completely, make the pH of the solution between 6.10~6.30, add 0.03% g / mL of medicinal activated carbon, stir and absorb for 30 minutes, filter through 0.45μm microporous membrane to remove carbon, add the filtrate for injection Water to 2000mL, fully stirred for 20 minutes to mix the solution evenly, and the prepared solution was sterilized by terminal filtration through a 0.22 μm microporous membrane to obtain the filtrate; put the filtrate into 1000 bottles of injection bottles, and the Put the injection bottle into the material tray and put it on the partition in the freeze-drying box in time, and pre-freeze: the temperature is -45°C, and the time is 4 hours; af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com