Oryzanol composition and its preparation method
A composition, oryzanol technology, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of slow onset, pain, poor patient compliance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
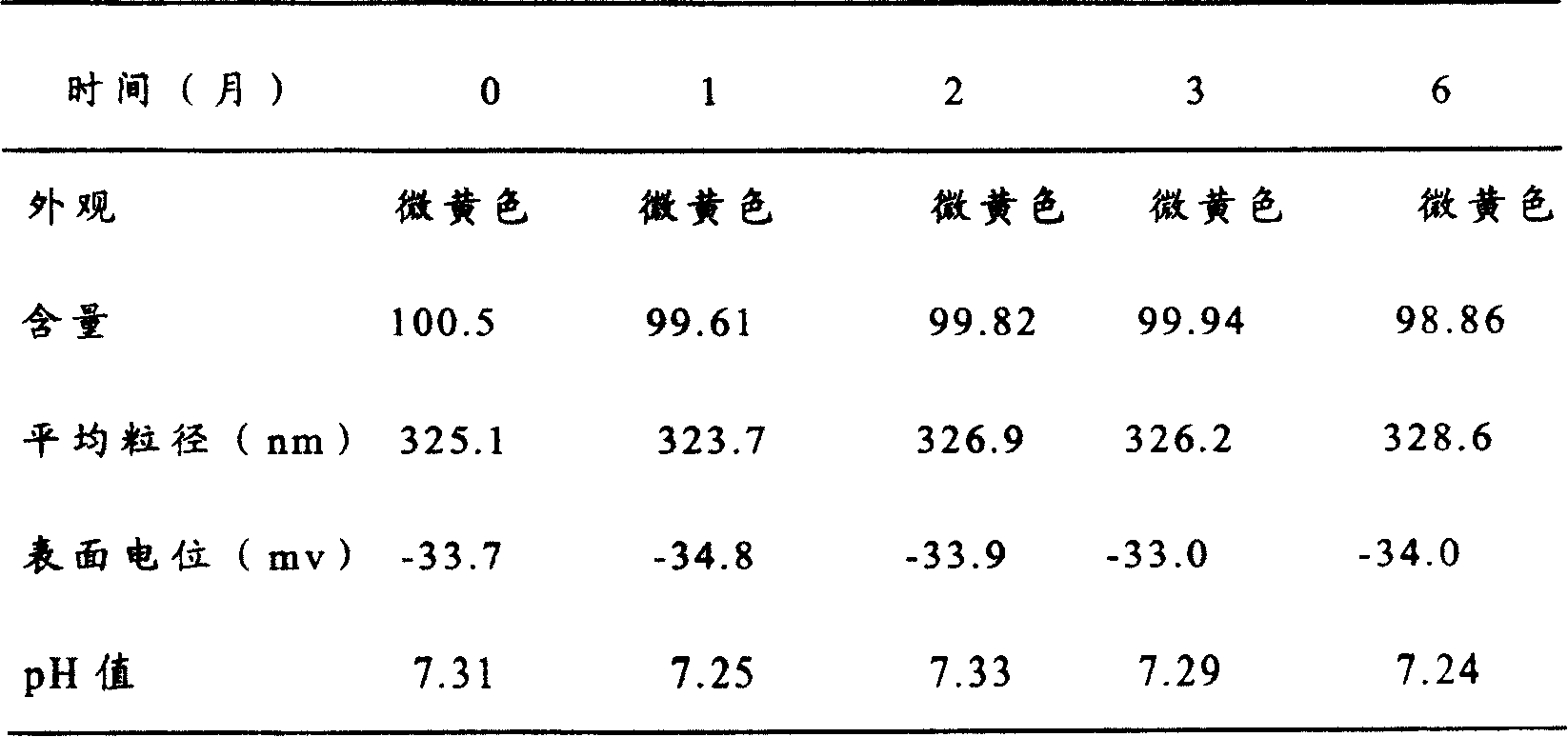
[0063] Add 30g of soybean oil for injection and 4.5g of oryzanol into the container, place the container in an oil bath, heat to 100°C, stir until the drug dissolves, cool to 80°C, and add 11g of soybean lecithin. Vitamin E 0.1g, stirred until the phospholipids dissolve to form a uniform oil phase. Put 80ml of water for injection in another container, add 2.5g of glycerin, stir and dissolve at 80°C to form a water phase; add the oil phase to the water phase under stirring, continue stirring for 40 minutes to make colostrum, and use sodium hydroxide solution Adjust the pH to 8; add water for injection to 100ml, and use a high-pressure homogenizer or an ultrasonic probe to homogenize the colostrum until the particle size of the milk droplets passes the inspection. Filter through a filter, pass through nitrogen, dispense into 1ml ampoules, and seal. Use a rotary autoclave to sterilize at 100°C for 30 minutes.
[0064]The prepared fat emulsion was stored in a cool place at room ...
Embodiment 2
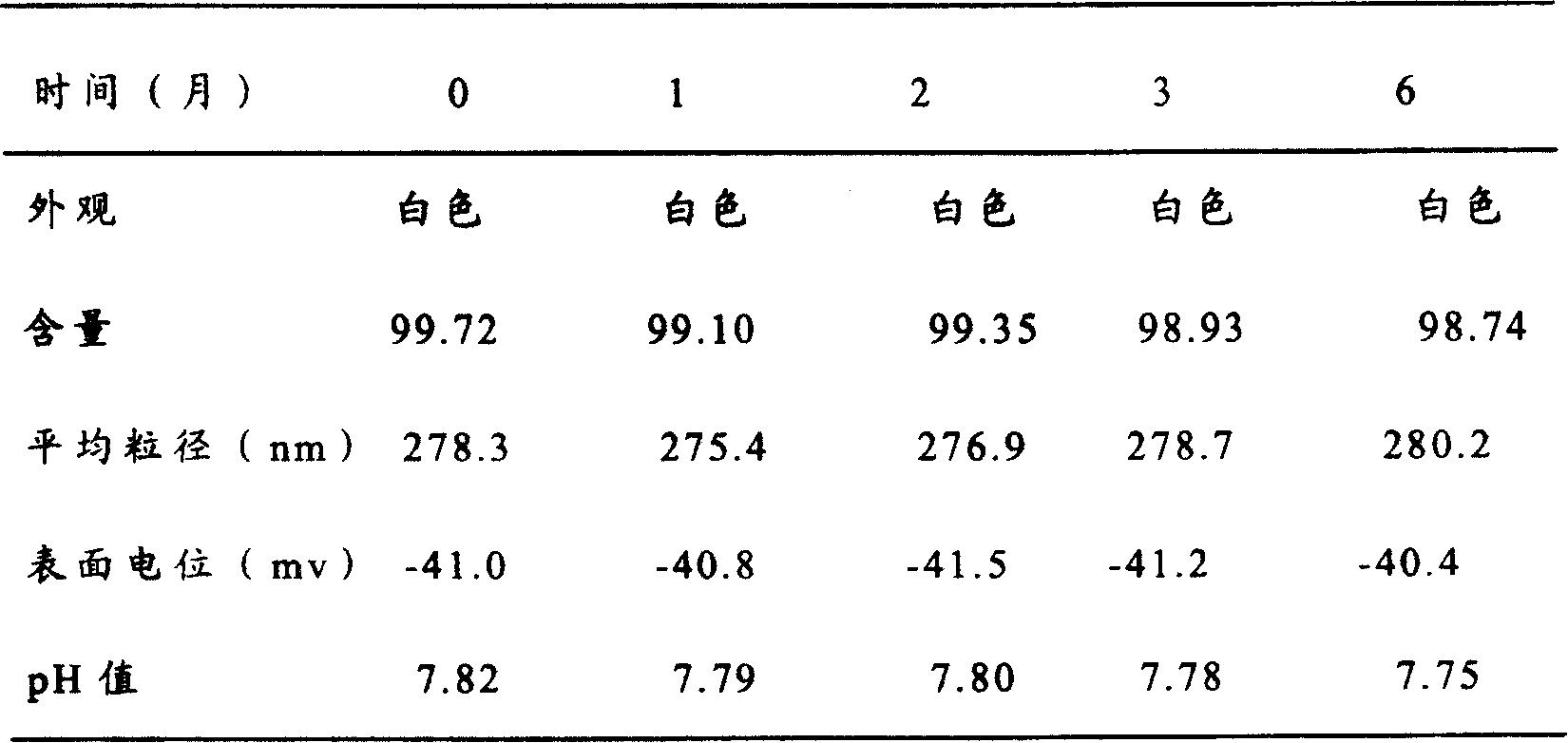
[0068] Add 7.5g of soybean oil for injection, 7.5g of olive oil, and 40mg of oryzanol into a container, place the container in an oil bath, heat to 70°C, and stir until the drug dissolves to form a uniform oil phase. Put 80ml of water for injection into another container, add 1.5g of egg yolk phospholipid, 0.5g of poloxamer, 0.5g of polyethylene glycol 12-position hydroxystearate, 5.0g of glucose, and 0.2g of sodium bisulfite in Stir and dissolve at 70°C to form a water phase; add the water phase to the oil phase under stirring, continue stirring for half an hour to make colostrum, adjust the pH to 8.5 with sodium hydroxide solution; add water for injection to 100ml, and mix the colostrum with high pressure Homogenize with a quality machine or an ultrasonic probe until the particle size of the emulsion droplet is qualified. Filter through a filter, pass through nitrogen, fill and seal. Use a rotary autoclave to sterilize at 115°C for 30 minutes.
[0069] The prepared fat em...
Embodiment 3
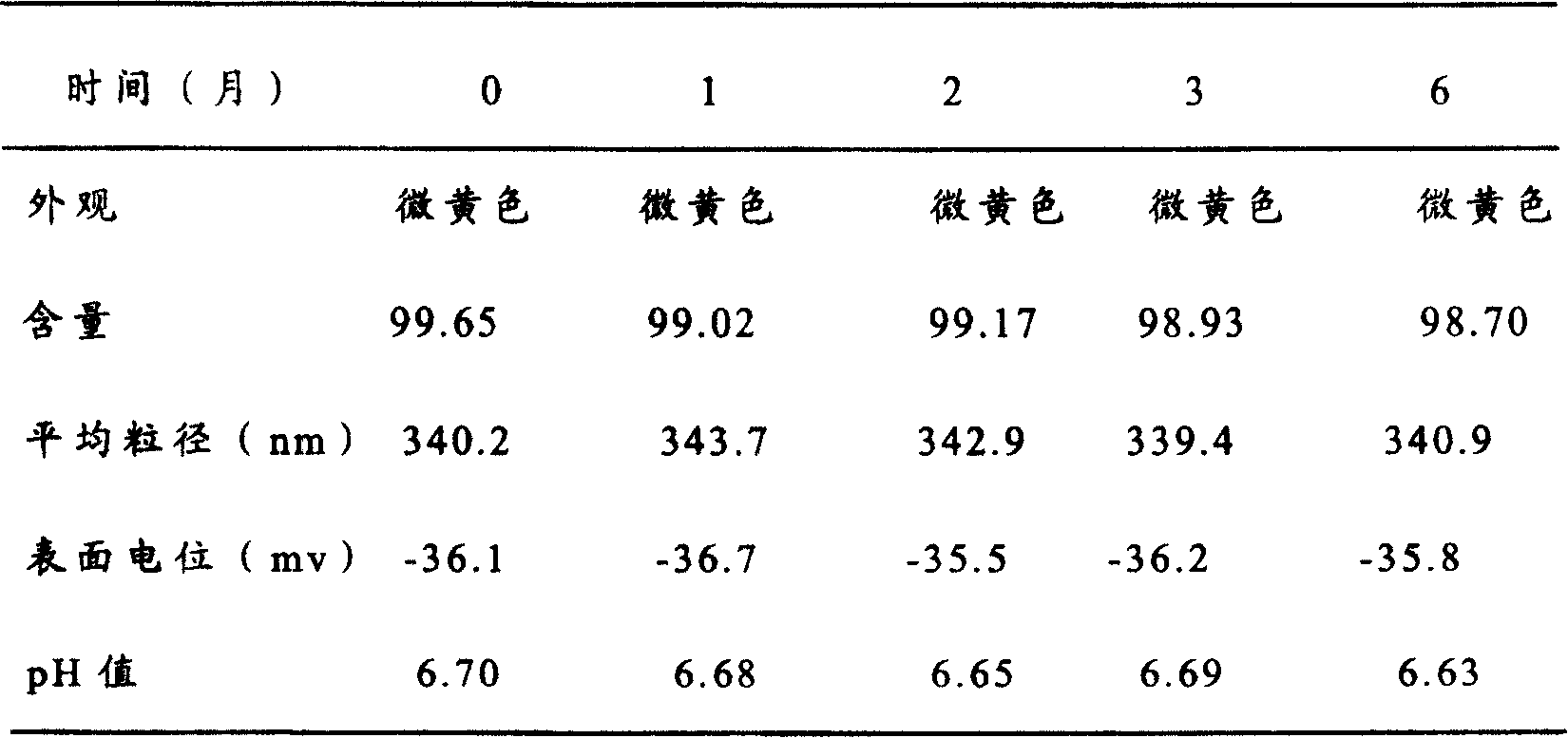
[0073] Add 10g of soybean oil for injection, 10g of medium-chain fatty acid ester, and 2g of oryzanol into the container, place the container in an oil bath, heat to 70°C, stir until the drug is dissolved, and dissolve 4.0g of 3-phosphatidylcholine with ethanol Add 2.0ml after dissolving, and evaporate the ethanol to form a uniform oil phase. Put 80ml of water for injection into another container, add 5.0g of sorbitol, 4.0g of poloxamer and stir to dissolve at 70°C to form a water phase; add the water phase to the oil phase under stirring, and continue stirring for 1 hour to prepare the initial Milk, add water for injection to 100ml, and adjust the pH to 7.0 with sodium hydroxide solution; use a high-pressure homogenizer or ultrasonic probe to homogenize the colostrum until the milk droplet size inspection is qualified. Filter through a filter, pass through nitrogen, dispense into 2ml ampoules, and seal. Use a rotary autoclave to sterilize at 100°C for 30 minutes.
[0074] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com