Compositions comprising ornithine and phenylacetate or phenylbutyrate for treating hepatic encephalopathy
A technology of phenylbutyric acid and phenylacetic acid, which can be used in medical preparations containing active ingredients, drug combinations, organic active ingredients, etc., and can solve problems such as shortening lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
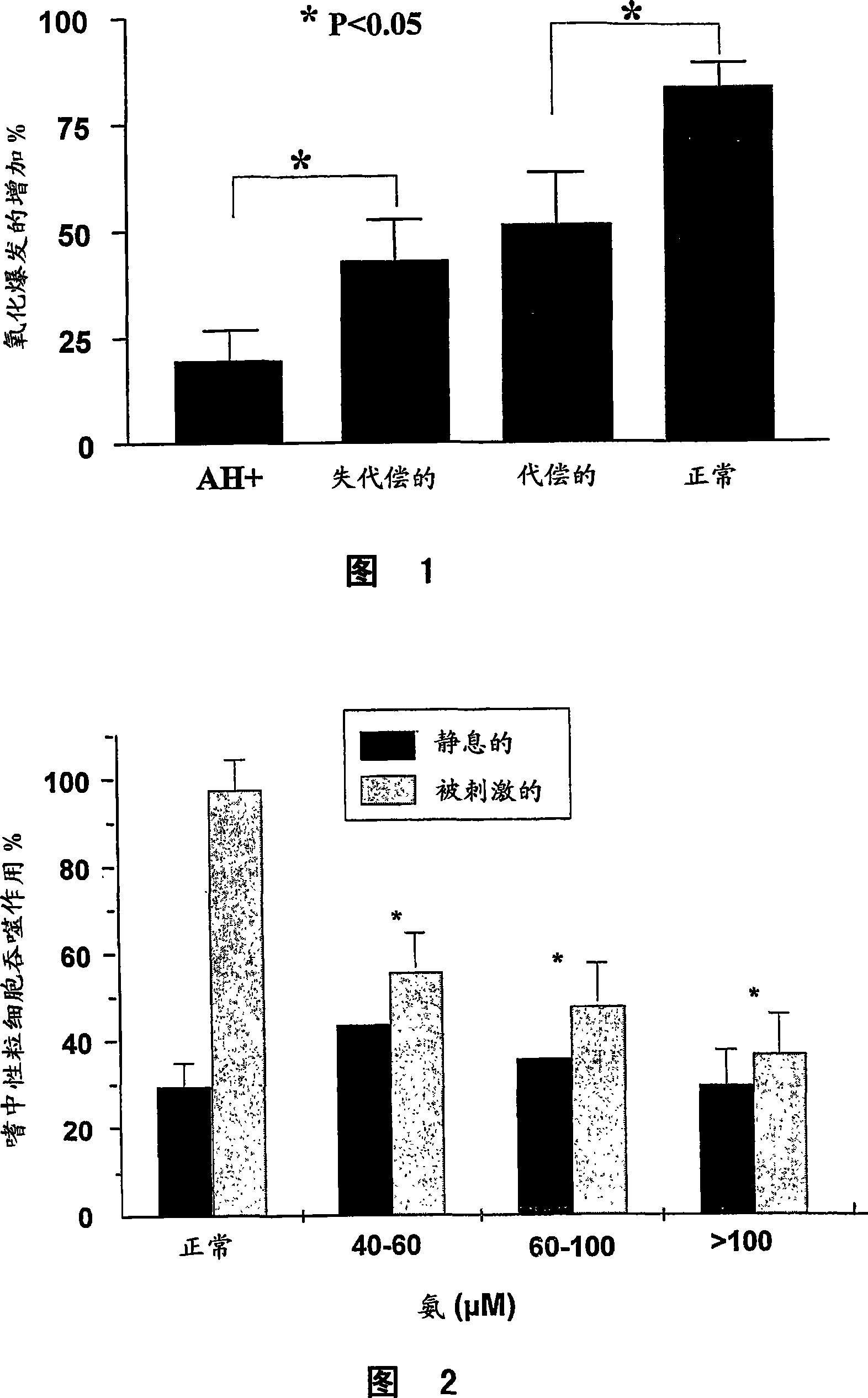
[0108] Example 1: Altered neutrophil function in patients with cirrhosis and progression with progressive liver disease change
[0109] Methods for Measuring Neutrophil Phagocytosis and Oxidative Burst
[0110] Phagocytosis assay: Heparinized whole blood was incubated with opsonized FITC-labeled E. coli and CD16. Cells were then analyzed by flow cytometry (FACScan Becton Dickinson), gating by forward and side scatter, followed by phycoerythrin (PE) [Immunotech, Marseille, France] fluorescent dye expression. Assess to identify CD16 positive cells. The gated cell populations were then evaluated for the presence of FITC-labeled bacteria.
[0111] Phagocytic Burst: Heparinized whole blood was incubated with an opsonized E. coli suspension to elicit an oxidative burst. The substrate solution was added to measure the conversion of dihydrorhodamine (DHR) 123 to the fluorescent source compound rhodamine (R) 123 . Reactions were stopped and fixed, then incubated with CD16 antibo...
Embodiment 2
[0117] Example 2: Ammonia reduces the phagocytosis of neutrophils
[0118] Methods for Measuring Phagocytosis and Oxidative Burst of Neutrophils
[0119] As described in Example 1.
[0120] patients and methods
[0121] Blood was collected from healthy volunteers (n=15) and incubated with increasing concentrations of ammonia for 1 hour. The ability of neutrophils to phagocytose bacteria was determined by phagocytosis assay and neutrophil chemotaxis assay. IL-8 at 10 ng / ml was used in the neutrophil chemotaxis assay.
[0122] result
[0123] Phagocytosis of neutrophils (Fig. 2) and chemotaxis of neutrophils (Fig. 3) were significantly attenuated as the concentration of ammonia in the culture was increased.
Embodiment 3
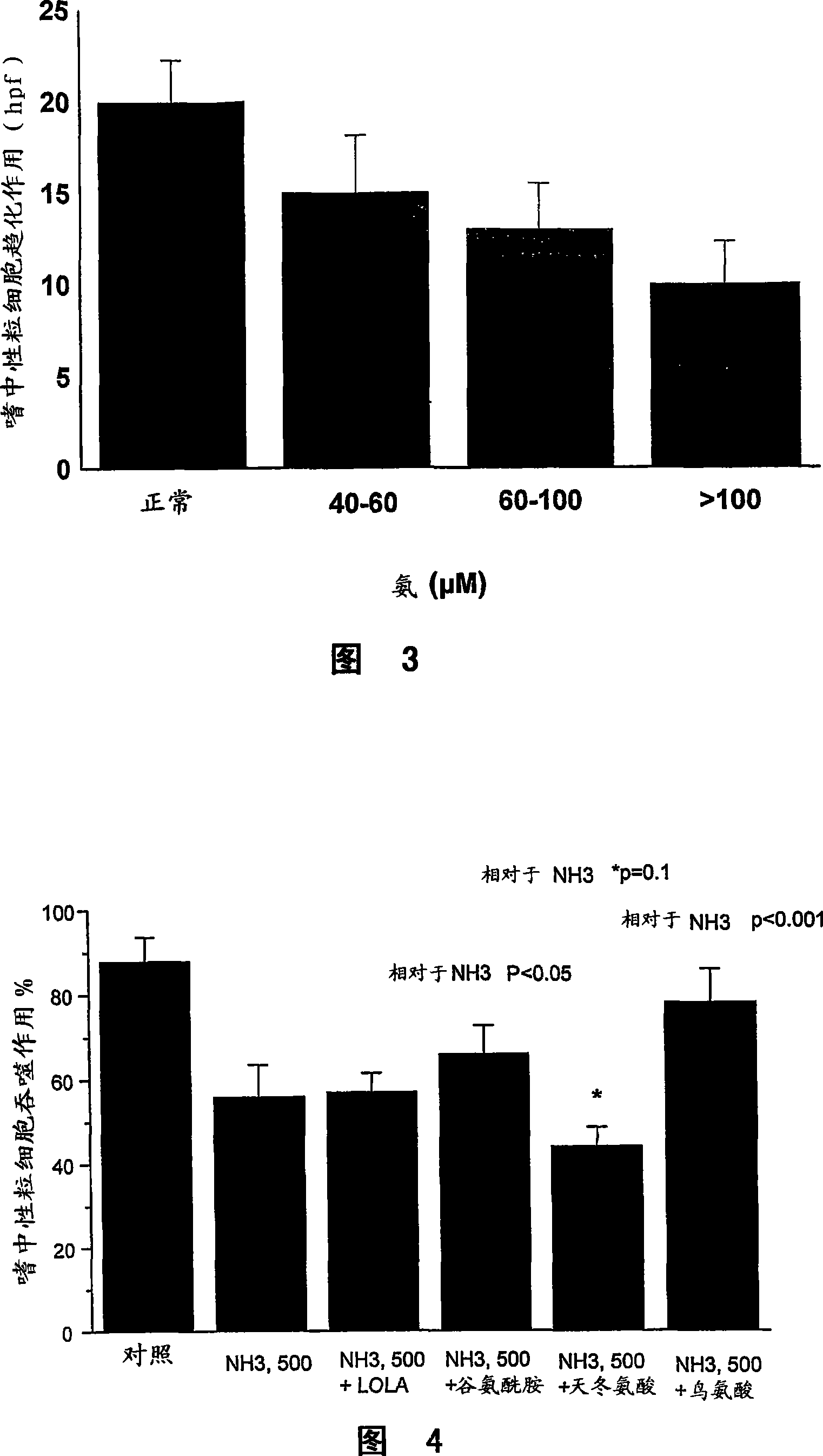
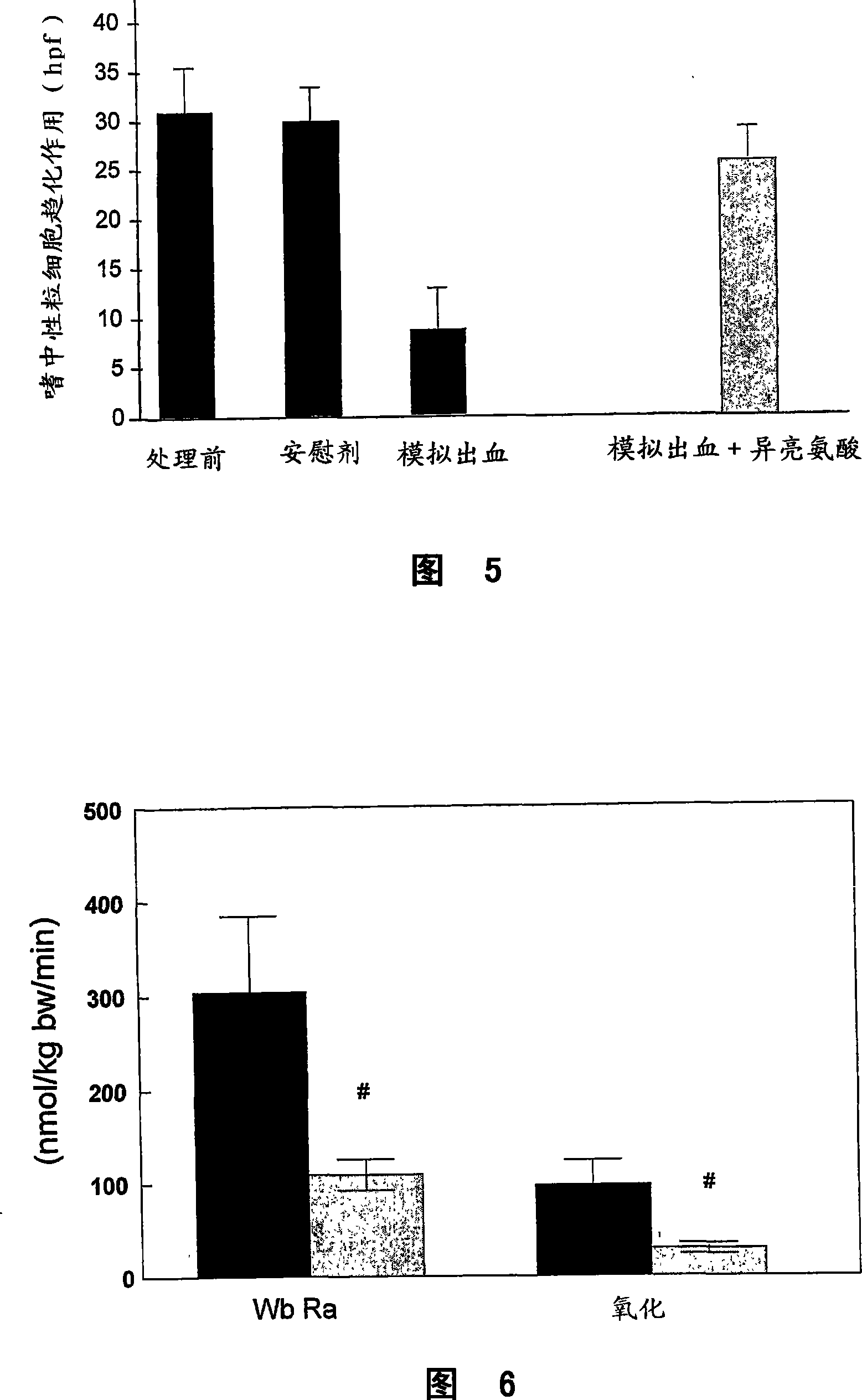
[0124] Example 3: The effect of ammonia on neutrophil phagocytosis can be reversed by intervention
[0125] Methods for Measuring Phagocytosis and Oxidative Burst of Neutrophils
[0126] As described in Example 1.
[0127] patients and methods
[0128] Blood was collected from healthy volunteers (n=15) and incubated with ammonia and selected amino acids for 1 hour. The ability of neutrophils to phagocytose bacteria was determined by phagocytosis assay.
[0129] result
[0130] We observed that ammonia-induced attenuation of neutrophil phagocytosis was partially reversed by ornithine and glutamine (Fig. 4). However, co-incubation with ammonia and aspartic acid worsened neutrophil phagocytosis, which remained unchanged when L-ornithine was used.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com