Patents
Literature
162 results about "Cranial aneurysm" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
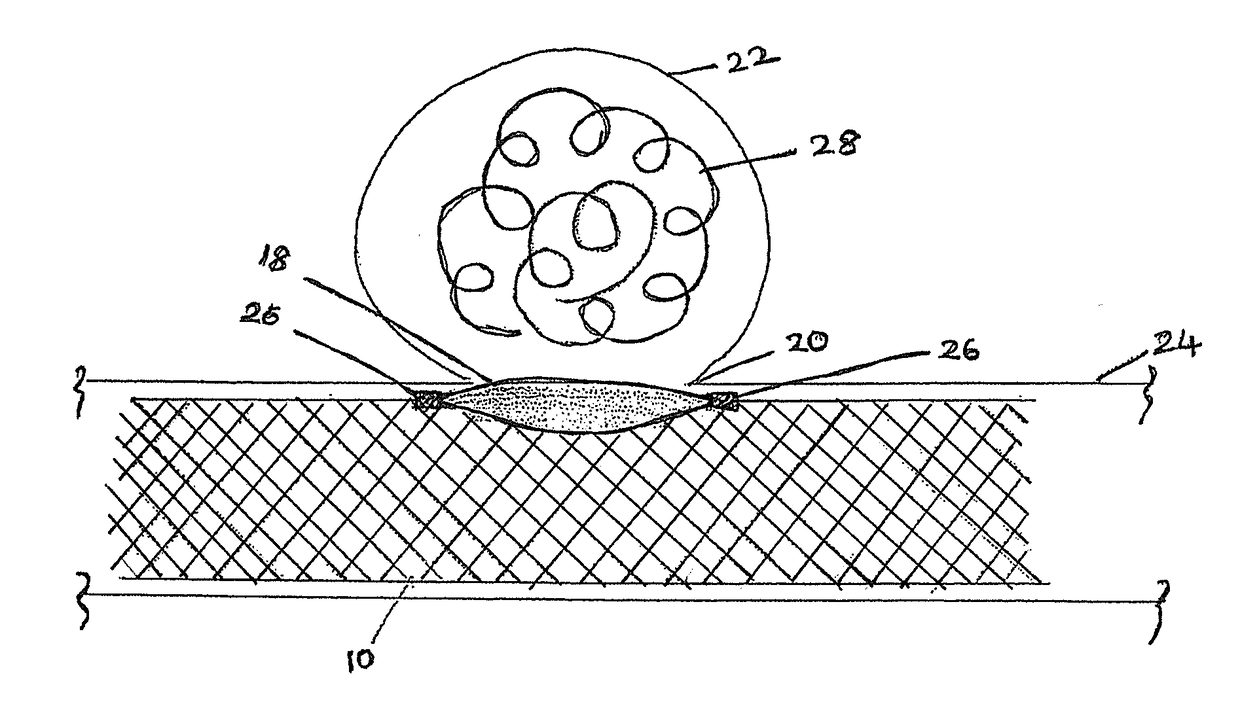
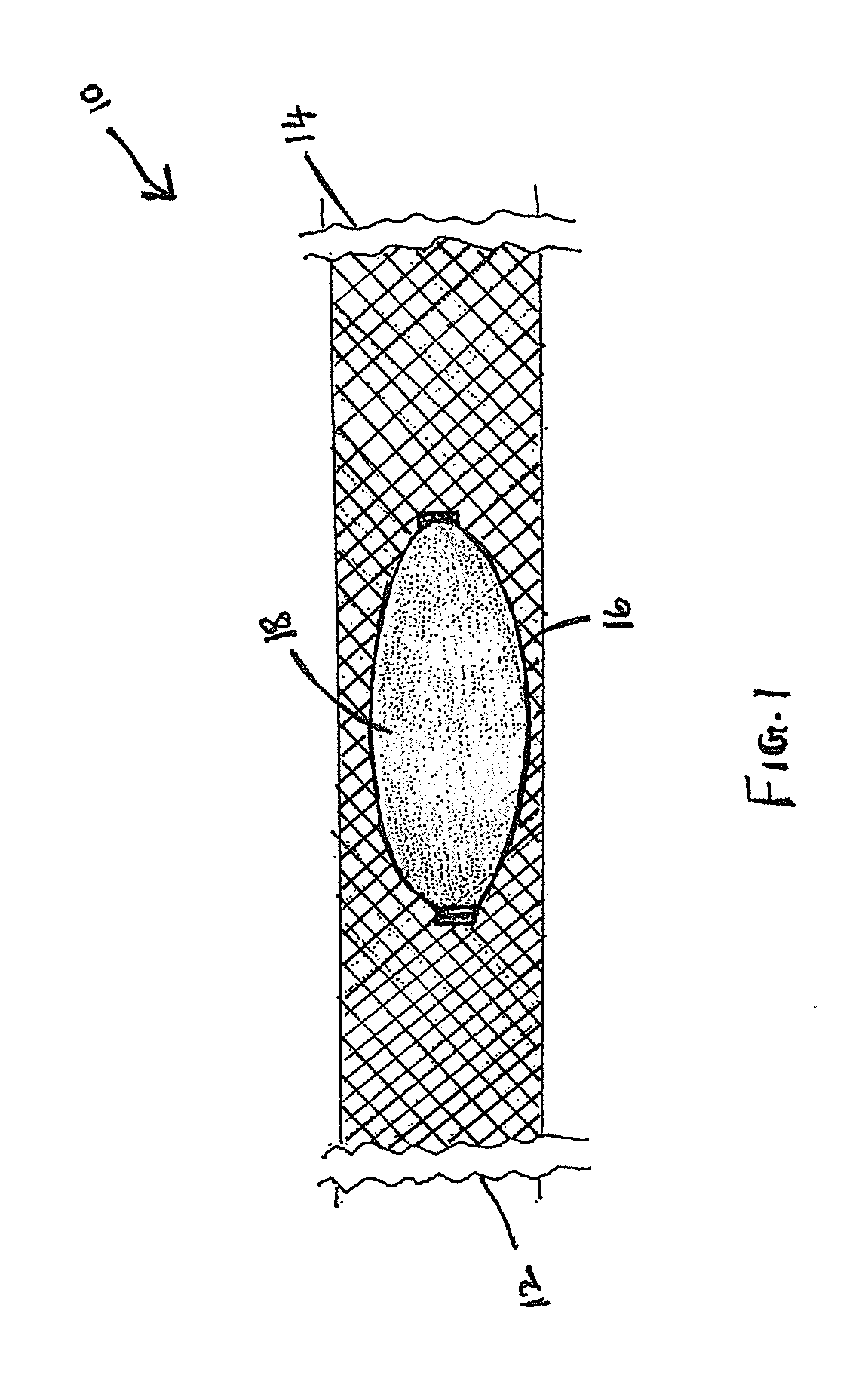
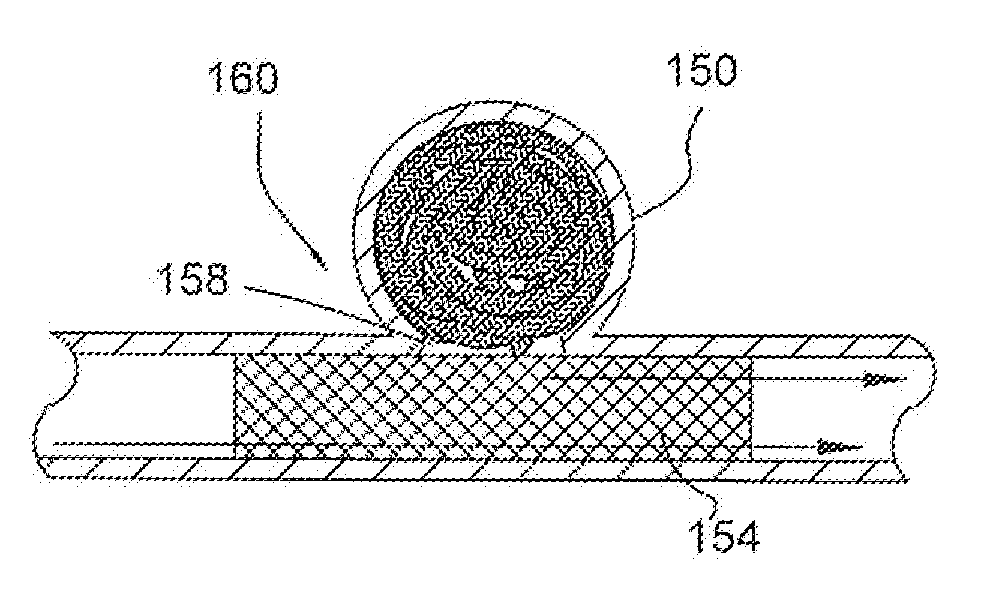
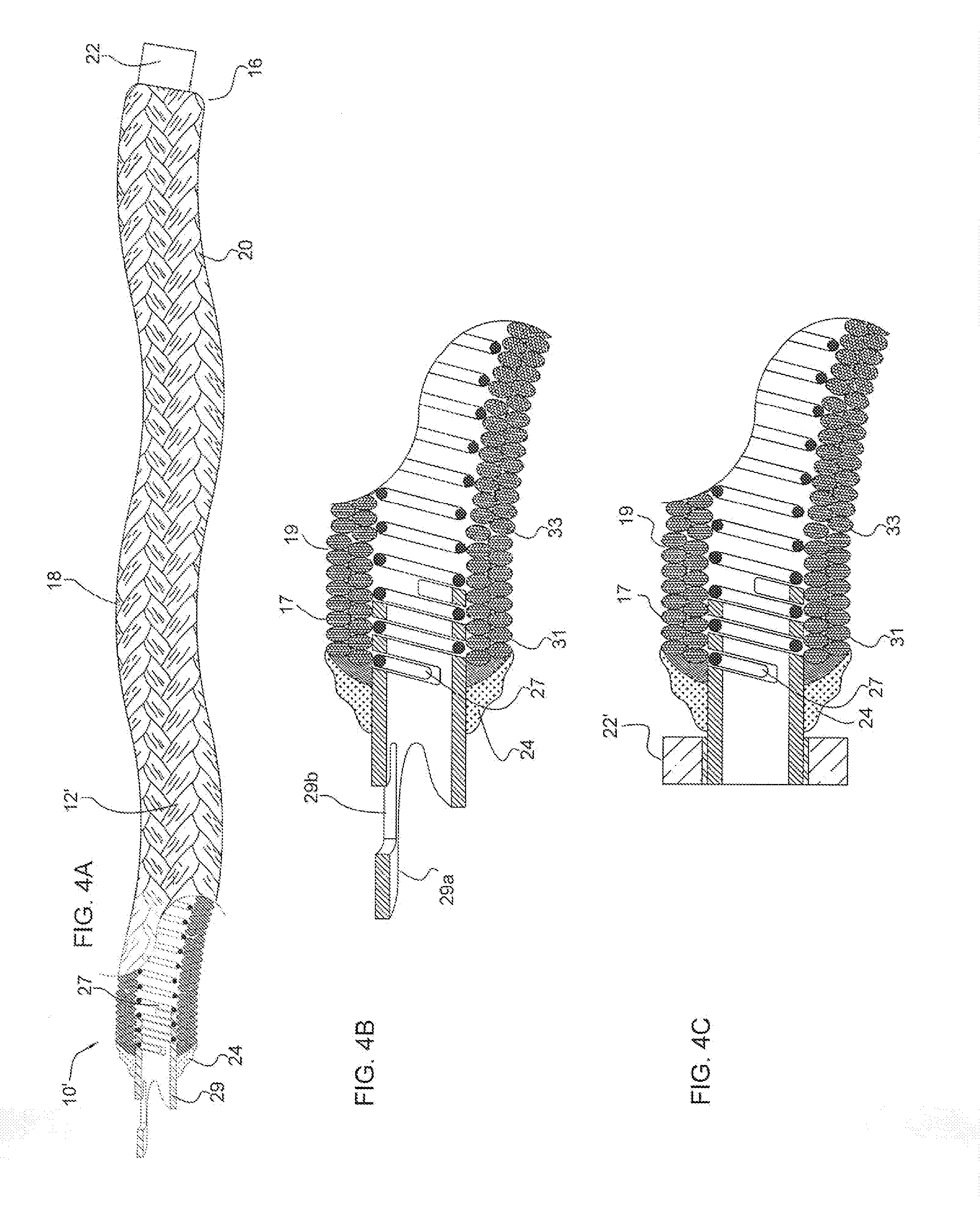
Cranial aneurysm treatment arrangement
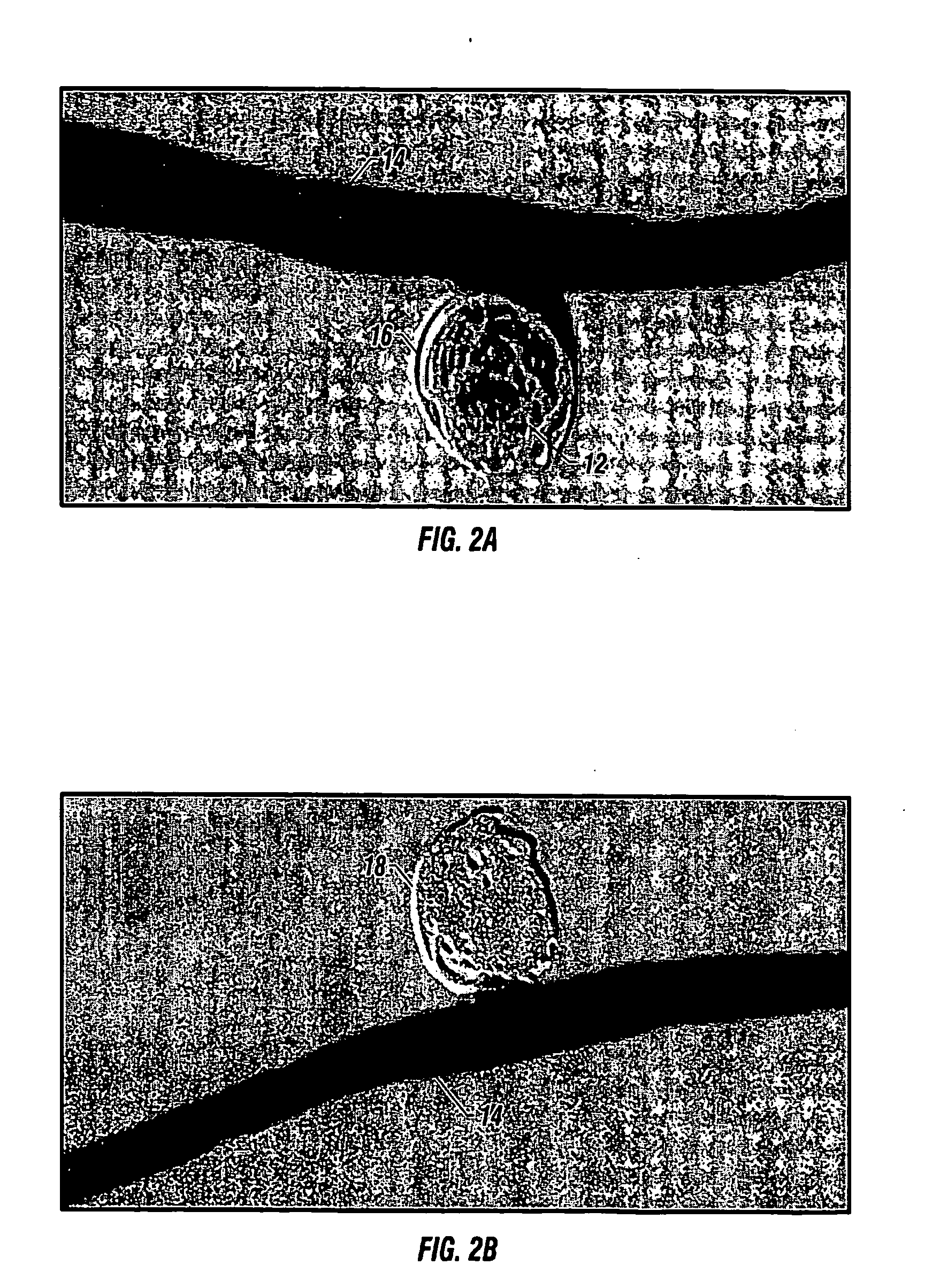
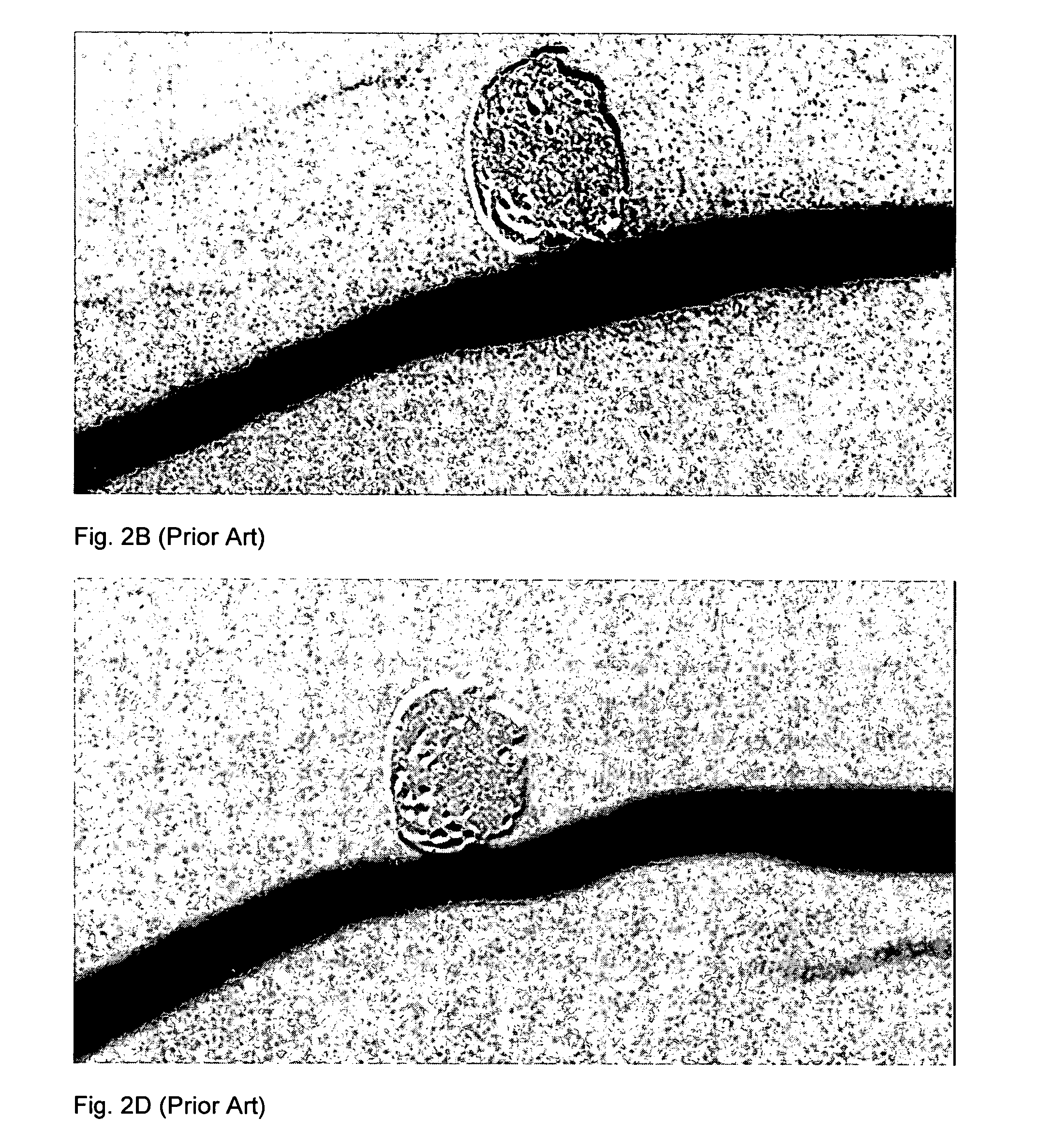
InactiveUS20060064151A1Minimize migrationMaximize flowStentsOcculdersInsertion stentAneurysm treatment
A stent for application within an aneurysm the stent comprising an elongated tubular member having a proximal end and a distal end. The stent has a proximal portion expandable from a first diameter to a second diameter. The distal end of the stent may be expandable to a third diameter, which third diameter is larger than the second diameter. The stent may be left disposed across the neck of the aneurysm.
Owner:GUTERMAN LEE R +1
Bioabsorbable polymeric implants and a method of using the same to create occlusions
A new embolic agent, bioabsorbable polymeric material (BPM) is incorporated to a Guglielmi detachable coil (GDC) to improve long-term anatomic results in the endovascular treatment of intracranial aneurysms. The embolic agent, comprised at least in part of at least one biocompatible and bioabsorbable polymer and growth factors, is carried by hybrid bioactive coils and is used to accelerate histopathologic transformation of unorganized clot into fibrous connective tissue in experimental aneurysms. An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location. Thrombogenicity of the biocompatible and bioabsorbable polymer is controlled by the composition of the polymer. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and bioabsorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly˜glycolic acid / poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides.
Owner:RGT UNIV OF CALIFORNIA
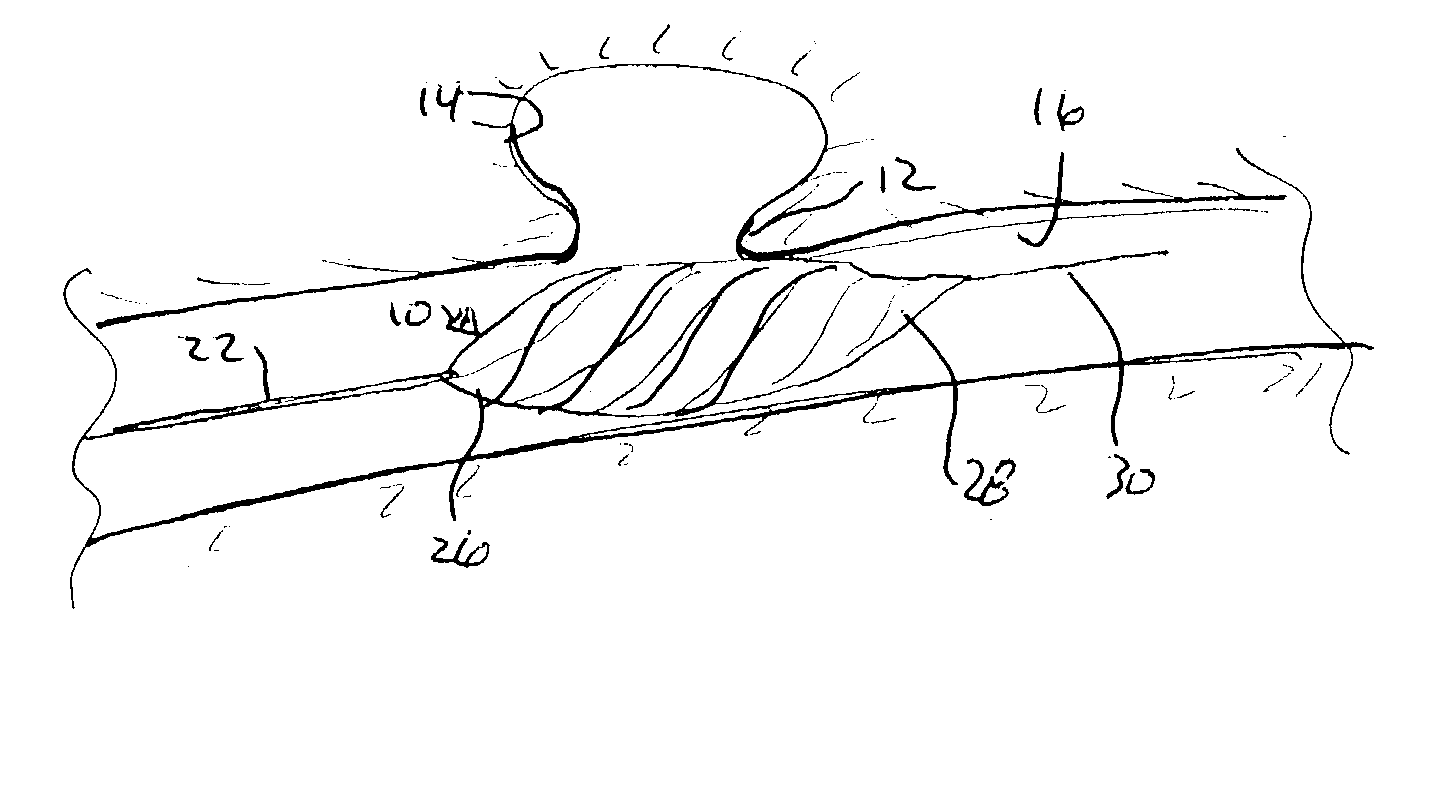
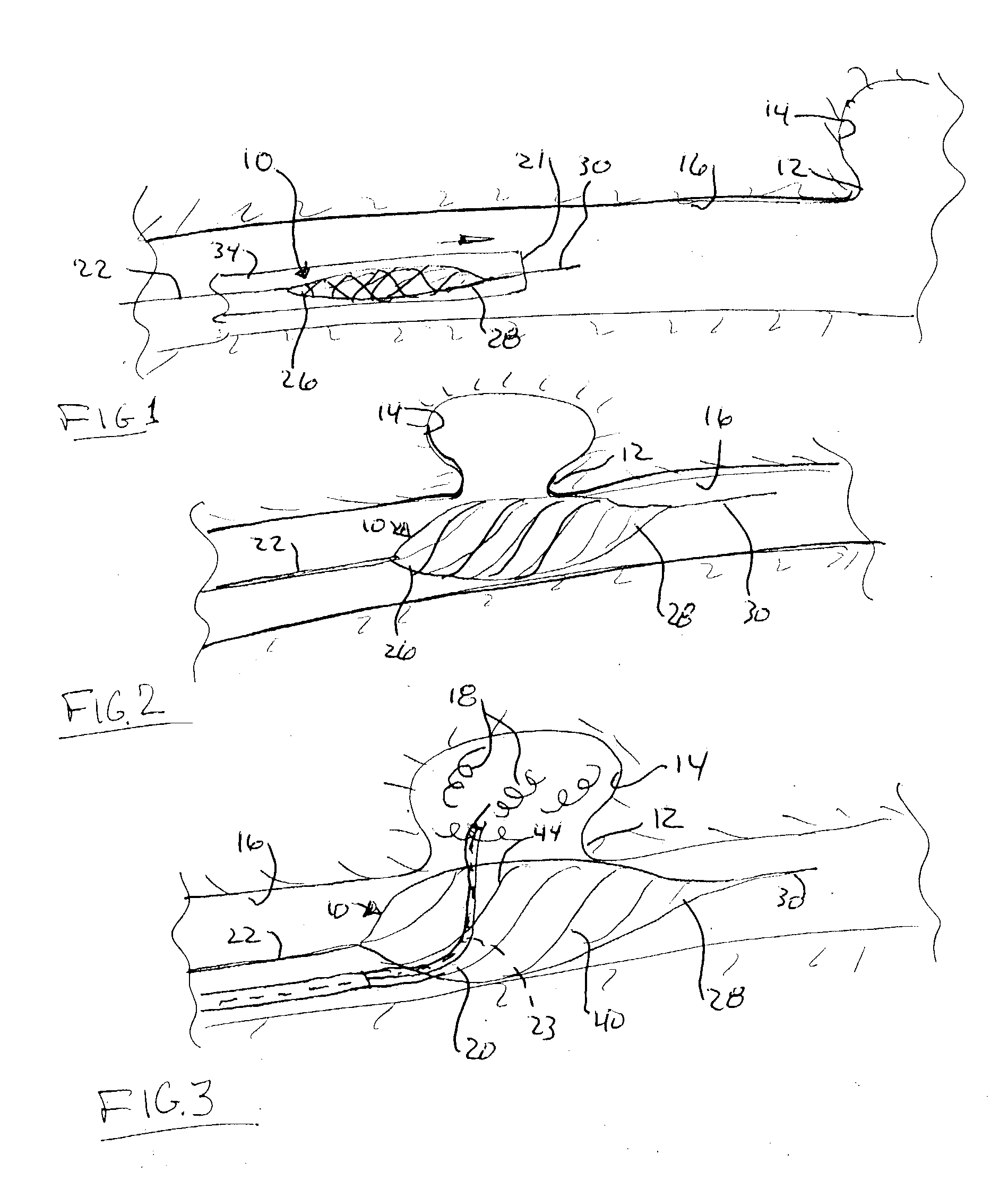
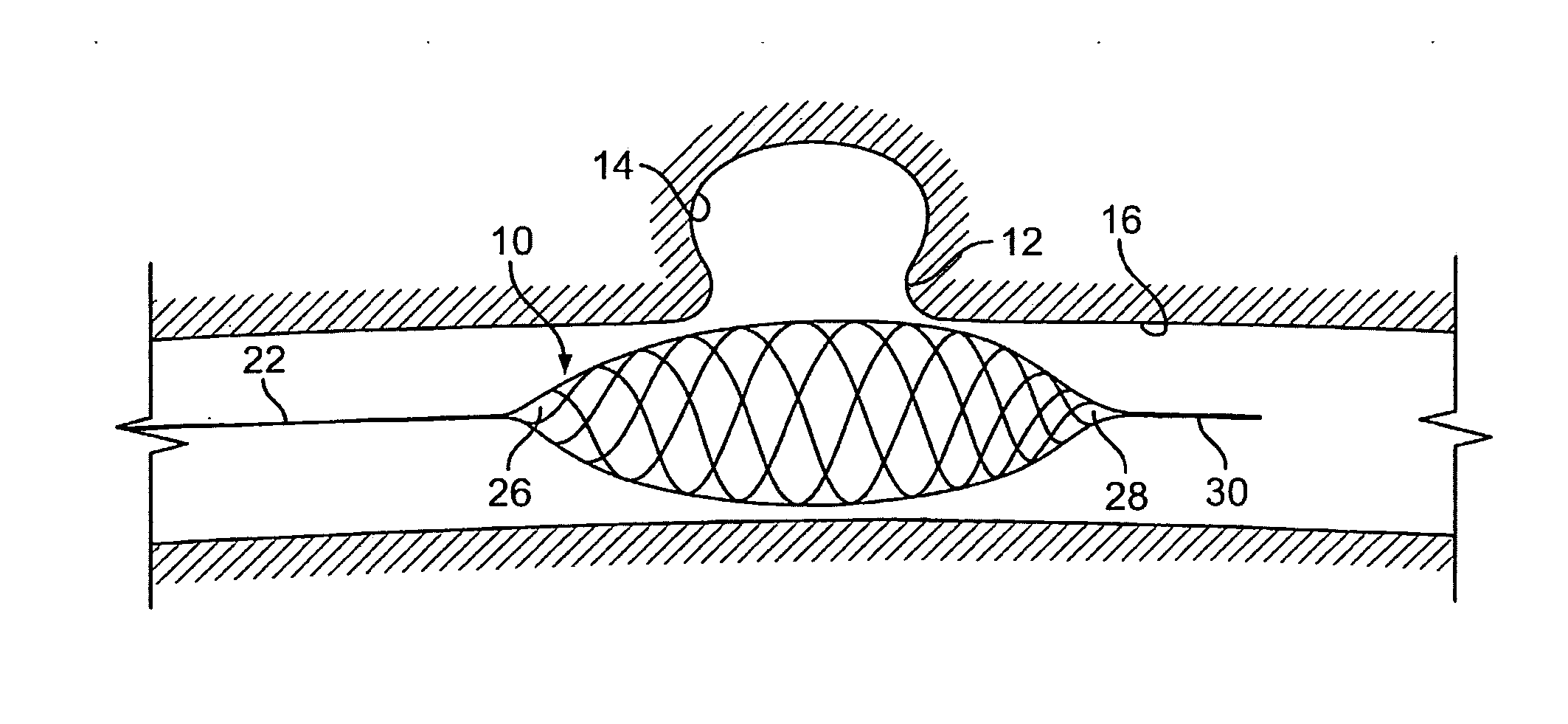
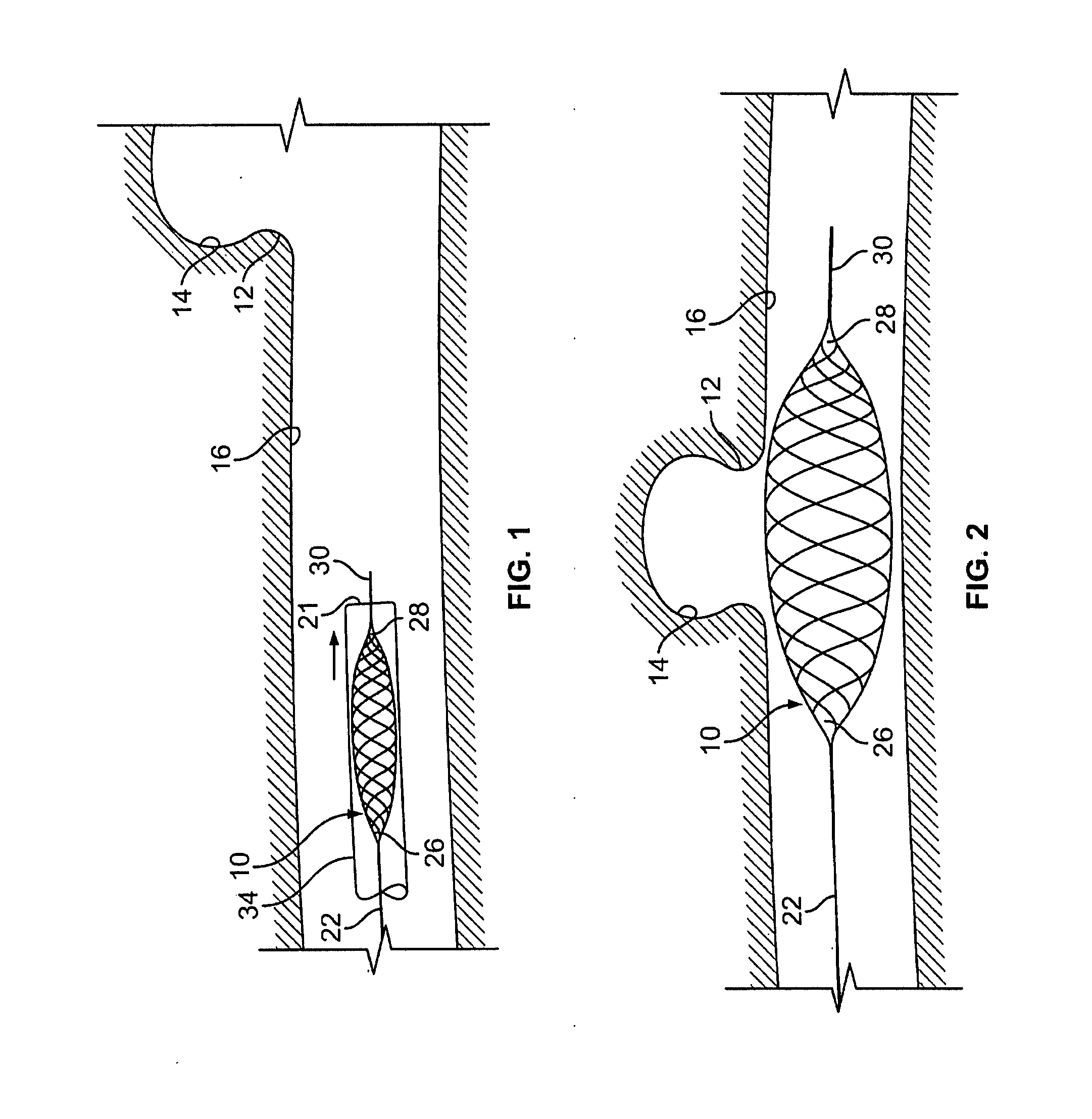
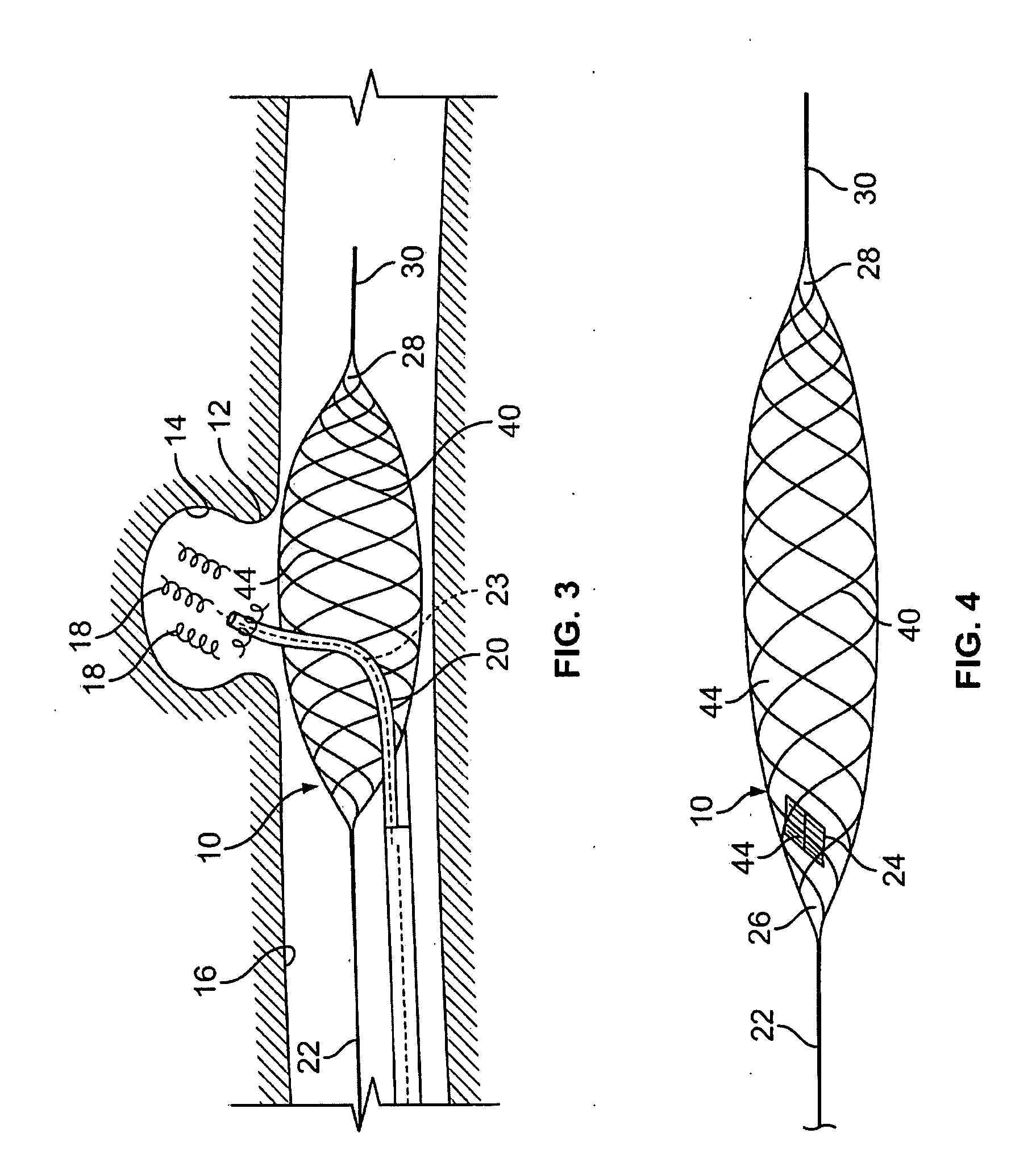
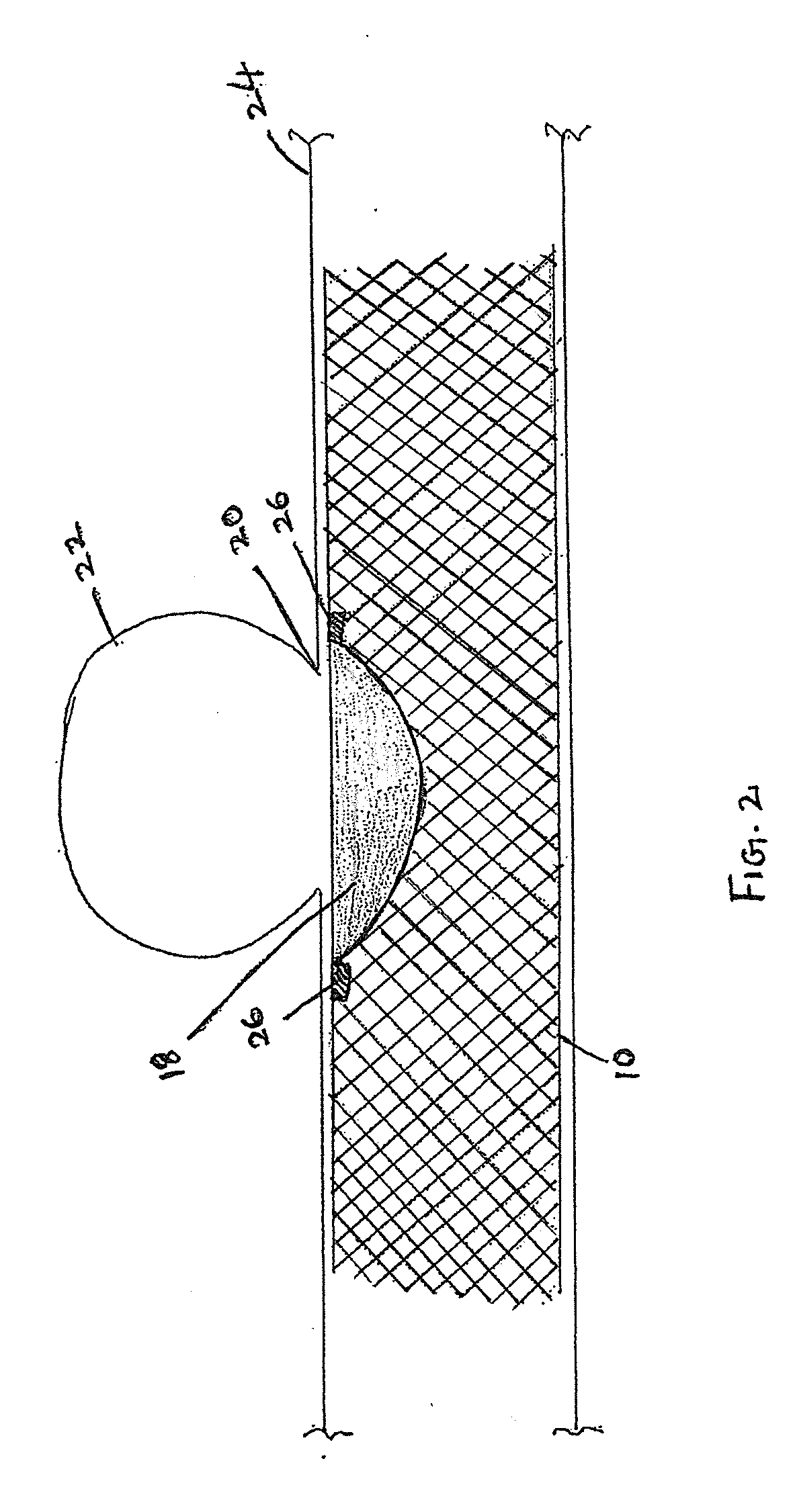
Endovascular aneurysm treatment device and delivery system
The present invention is directed to an intravascular treatment for intracranial aneurysms. The invention consists of a patch stent and, also, a patch stent delivery system allowing one to rotate the stent to align the patch with the neck of the aneurysm. A self-expanding nitinol framework holds the patch in place, a radiopaque agent allows the patch location to be visualized and a pusher tube that is mechanically locked into the unexpanded stent is used to push the stent out of the catheter with rotational and longitudinal control necessary to align the patch with the aneurysm. A self-expanding framework is used to support a patch at the neck of the aneurysm. The patch is designed to reduce the blood circulation in the aneurysm and the stagnant blood will clot or thrombus. The thrombus will stop any current blood leakage into the brain and will dramatically reduce the possibility of future leaks or potentially deadly ruptures. Over time the thrombus will be absorbed and the volume of the aneurysm will shrink reducing pressure on surrounding tissue.
Owner:HINES RICHARD ALLEN
Biodegradable polymer coils for intraluminal implants
An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location of a separable coil comprised at least in part of at least one biocompatible and absorbable polymer or protein and growth factors. Typically a catheter associated with the separable coil is used to dispose the coil into a selected body lumen. The biocompatible and absorbable polymer or protein is thrombogenic. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and absorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides. The biocompatible and absorbable protein is at least one protein selected from the group consisting of collagen, fibrinogen, fibronectin, vitronectin, laminin, and gelatin. In one embodiment the coil is composed of the biocompatible and absorbable polymer or protein with a radio-opaque material is disposed thereon. Alternatively, the coil is composed of a radio-opaque material, and the biocompatible and absorbable polymer or protein is disposed thereon. This apparatus may be positioned within intracranial aneurysms or any aneurysm in the body as well as within other body cavities.
Owner:RGT UNIV OF CALIFORNIA
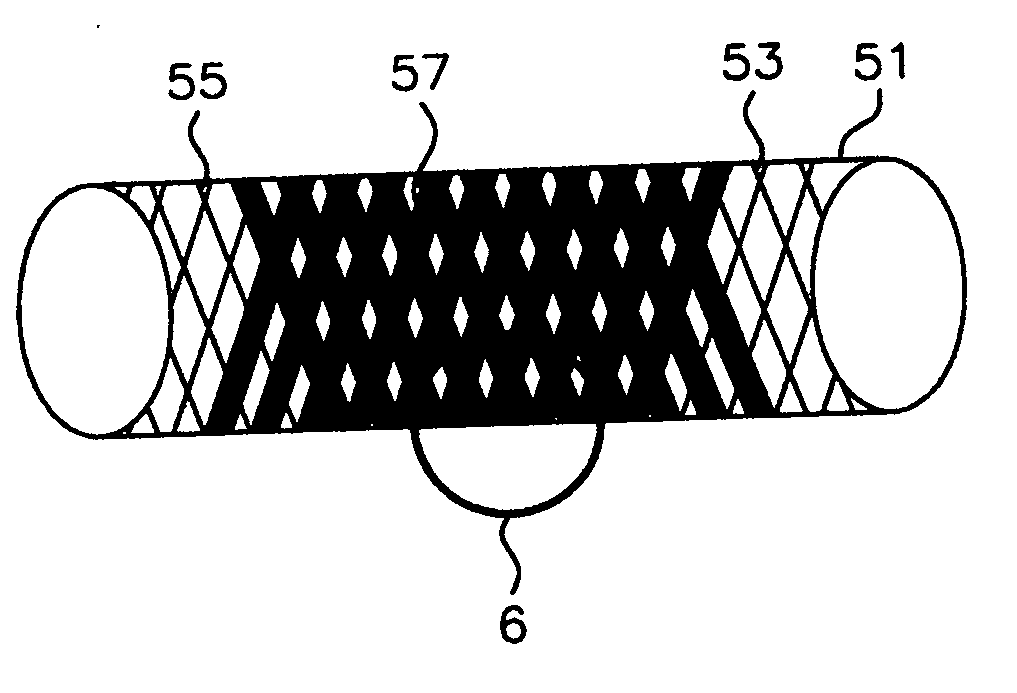
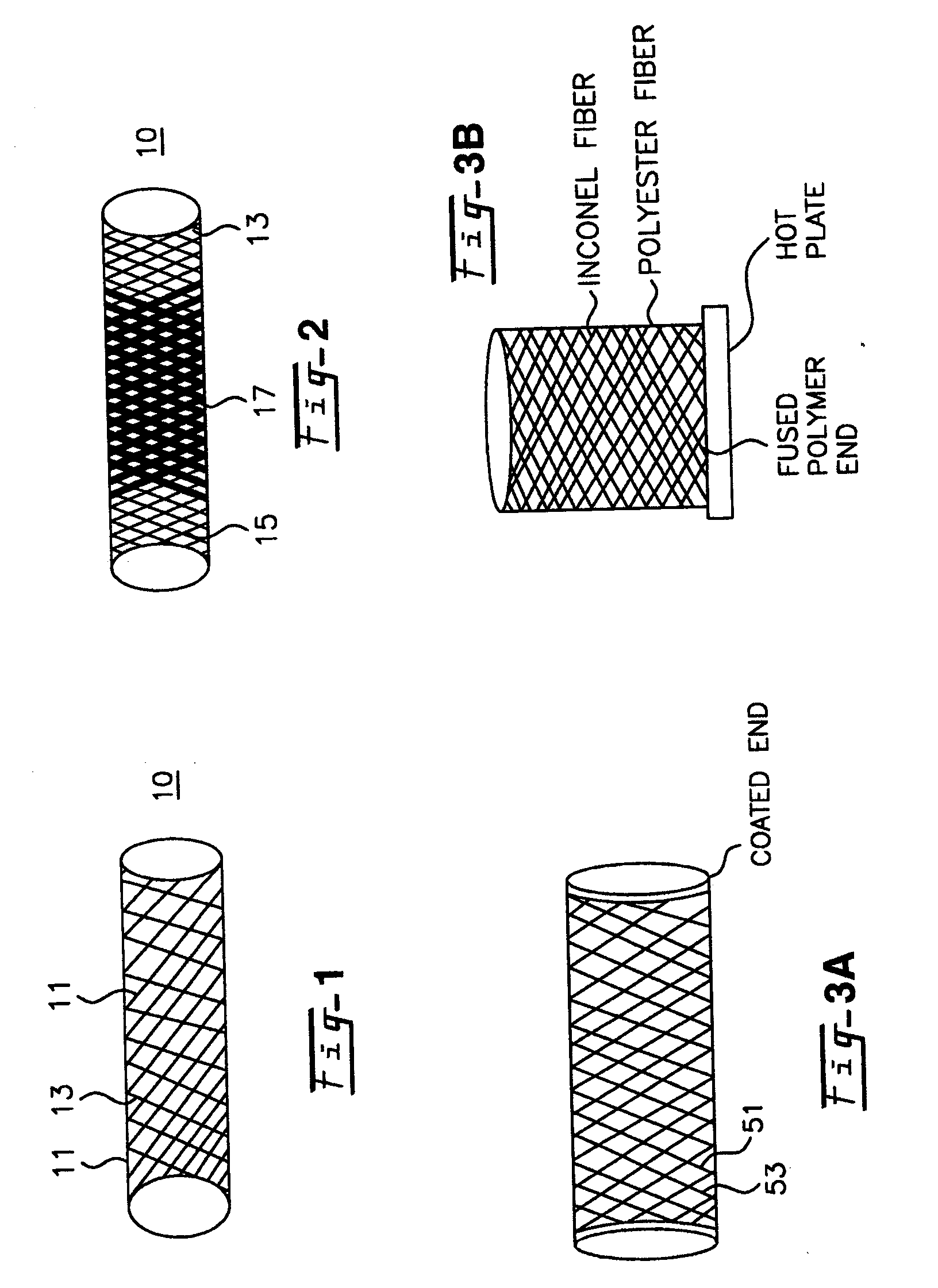
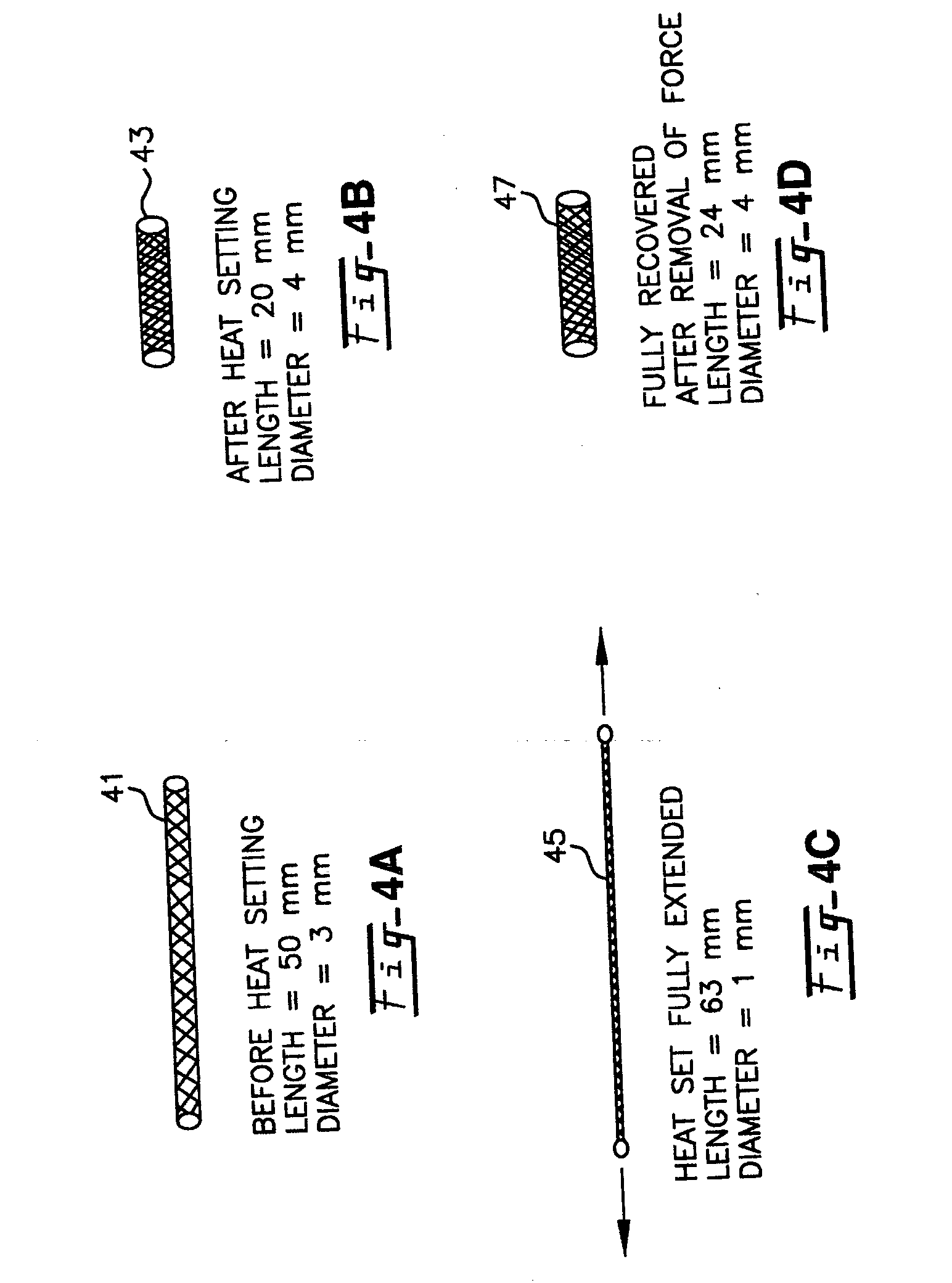
Blood flow diverters for the treatment of intracranial aneurysms
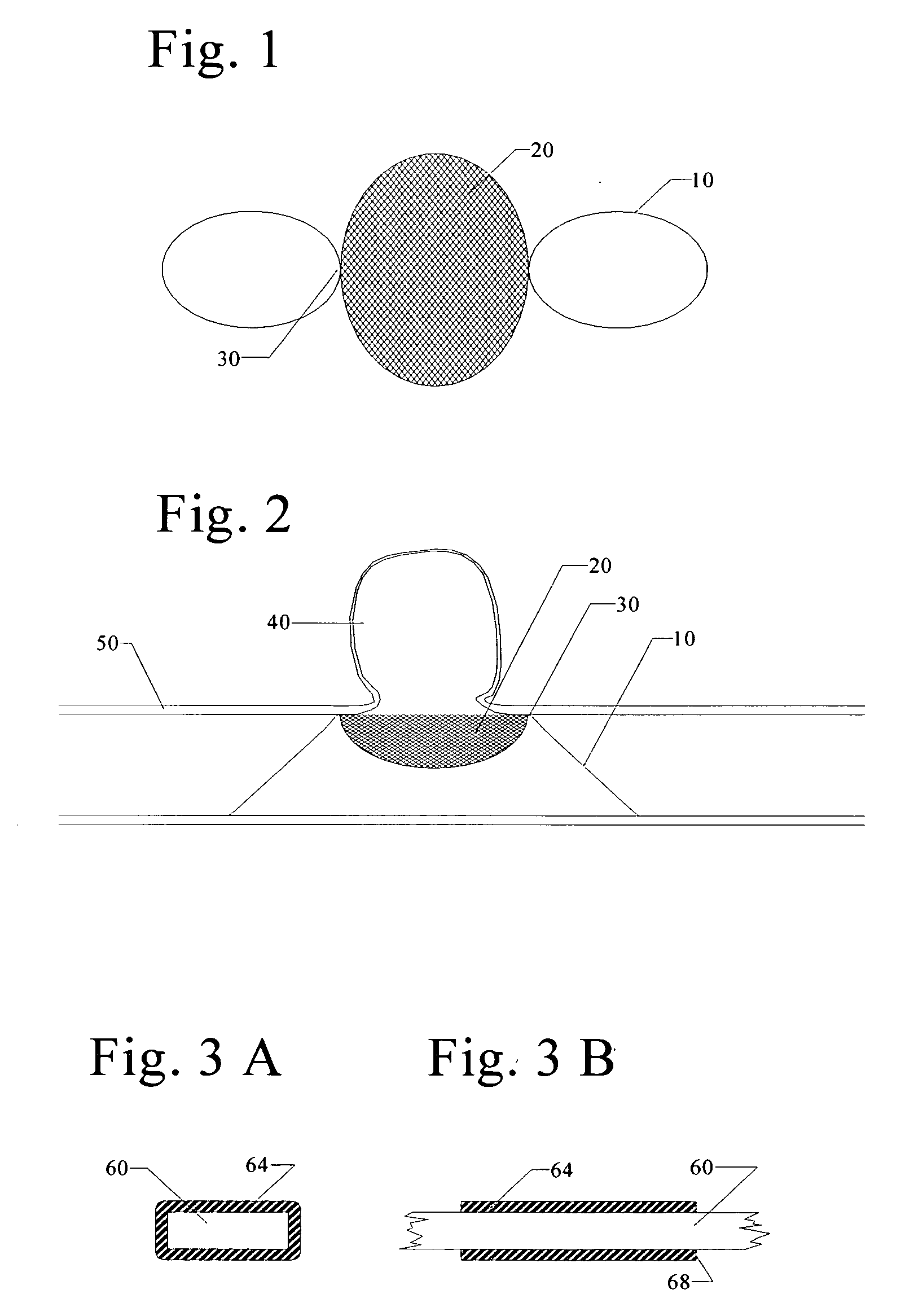
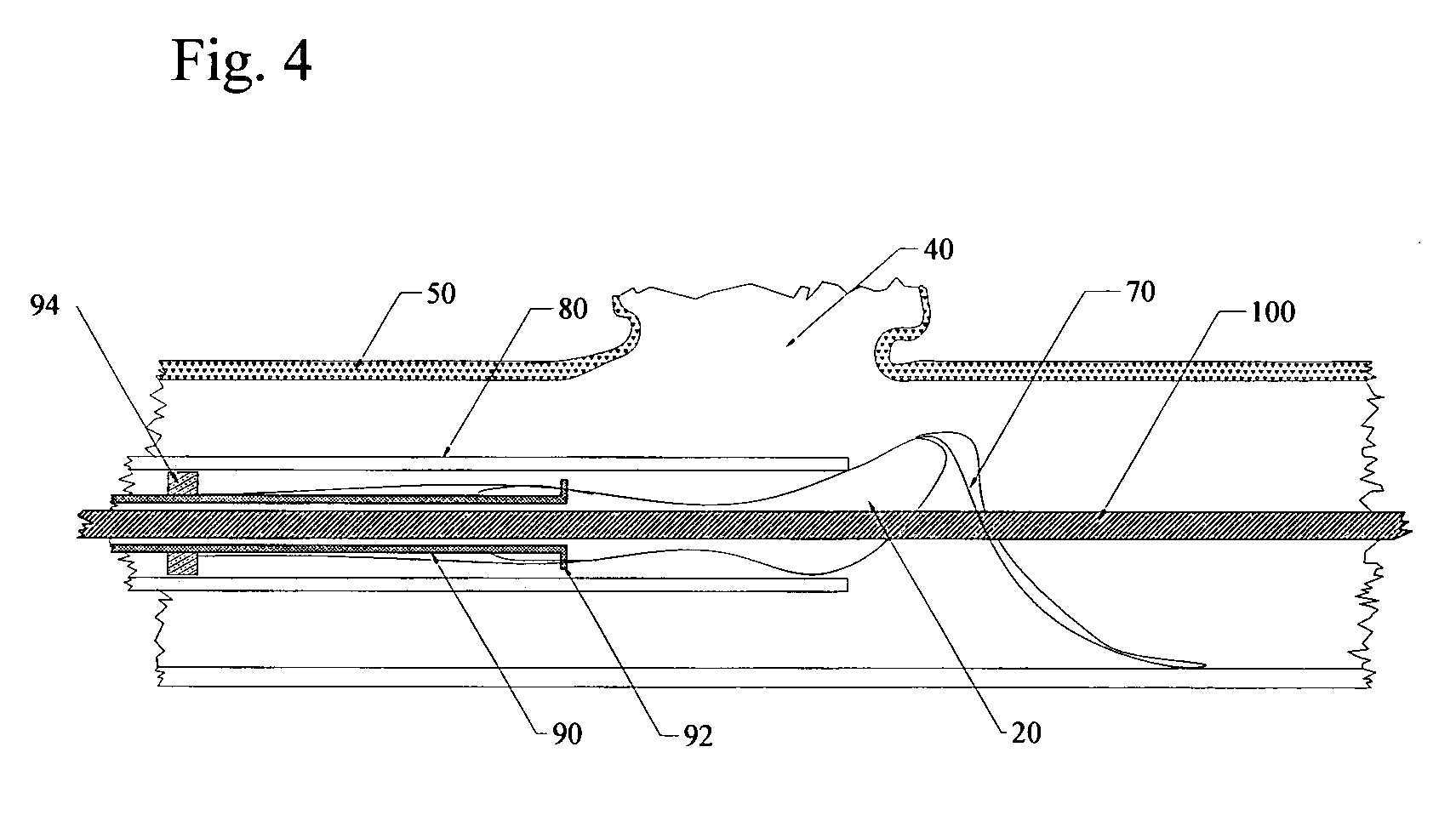
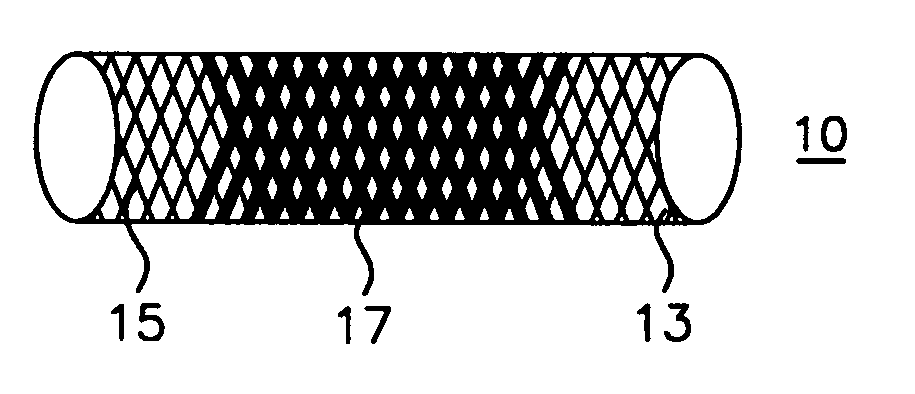
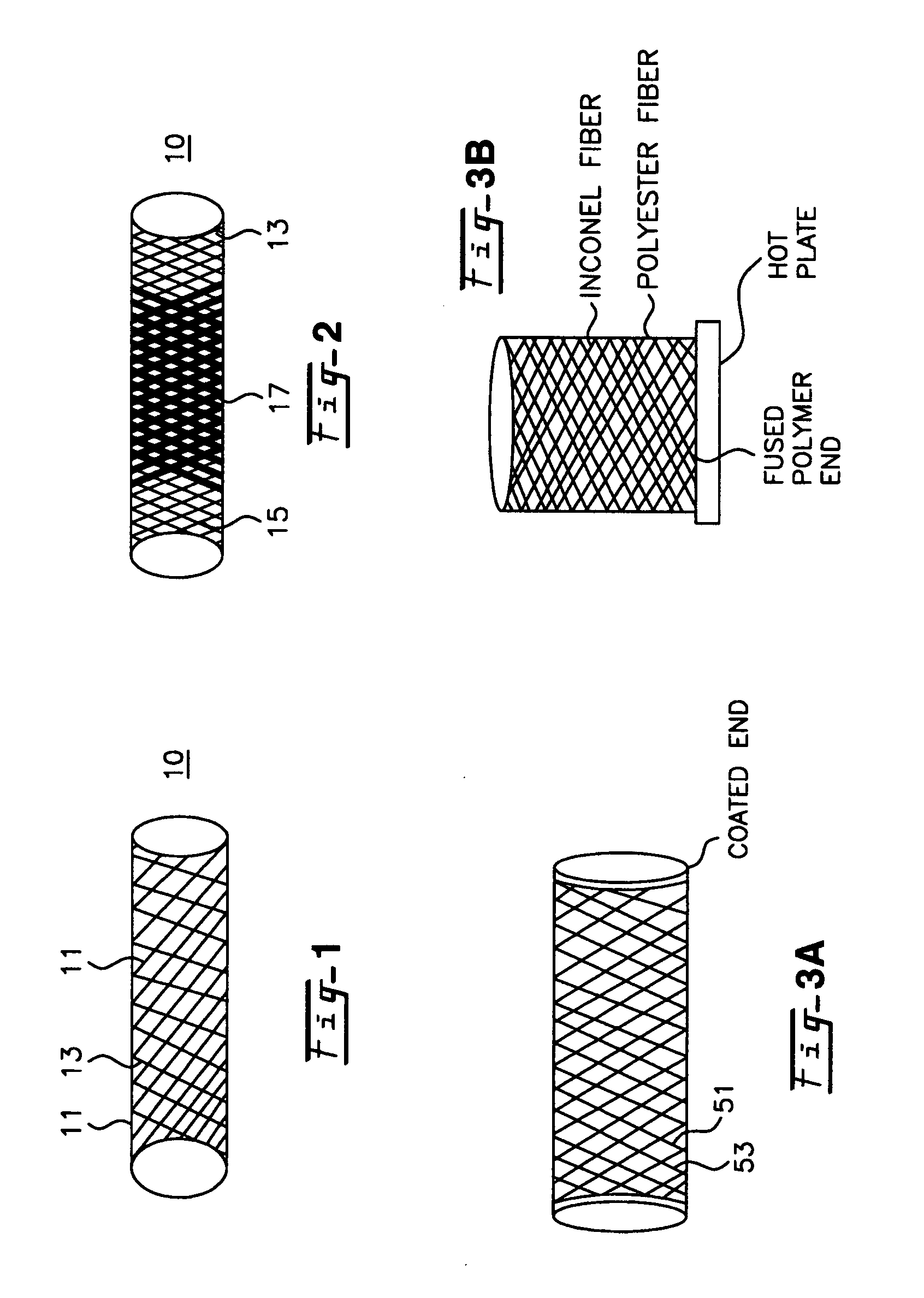
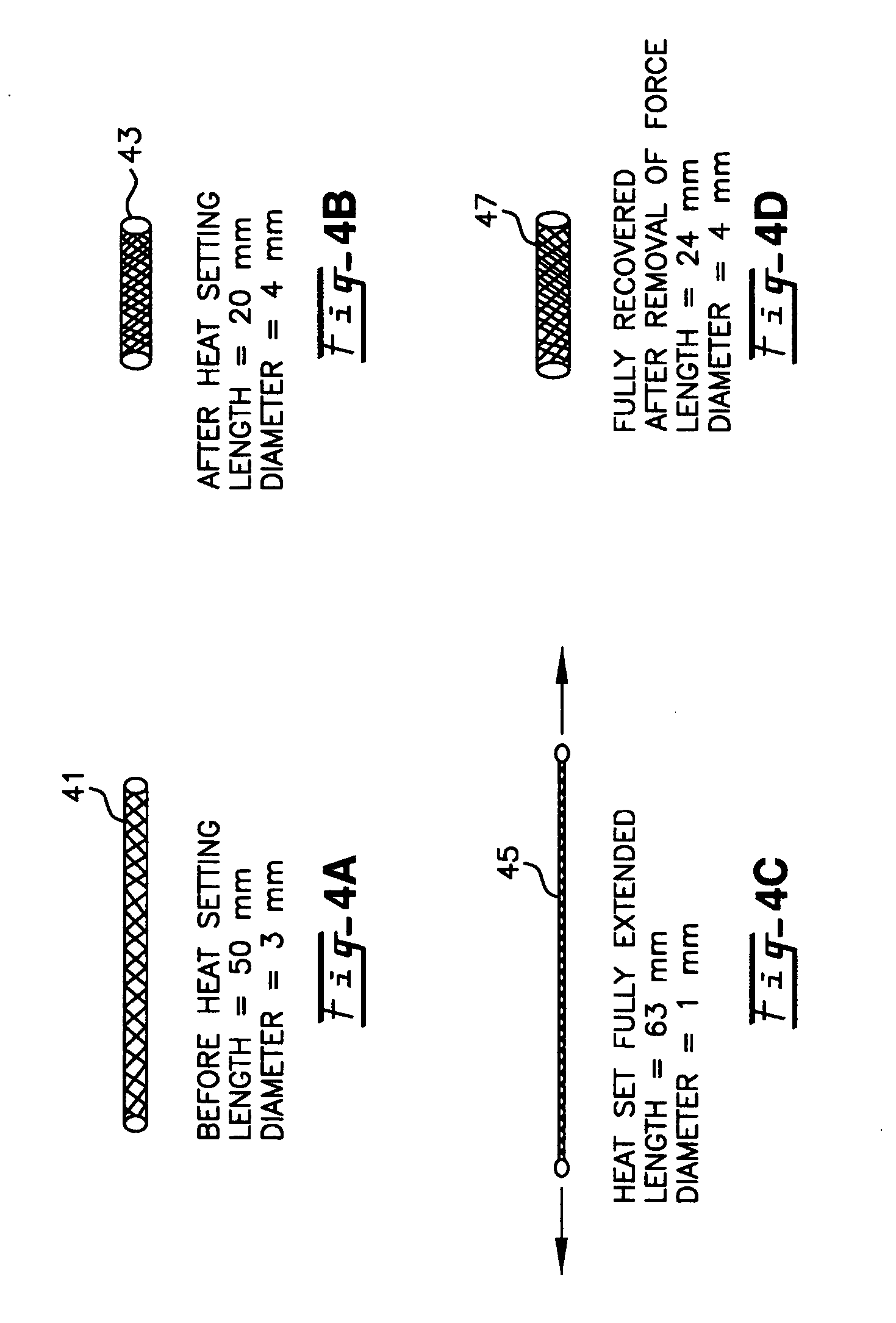
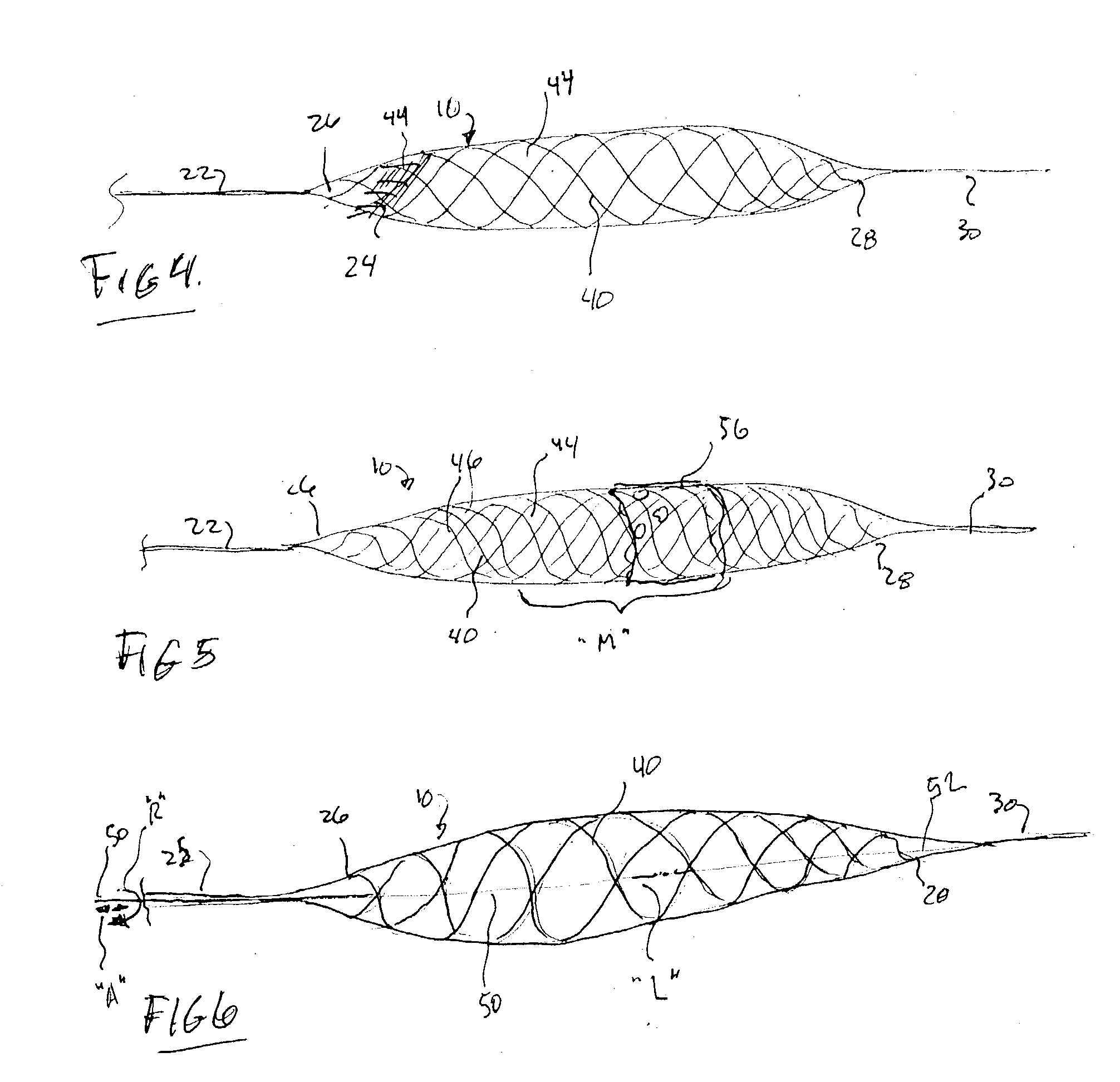
A blood flow diverter device for treatment of intracranial aneurysms, including a porous tubular member having a central portion and two ends. The member is of sufficient flexibility and body compatibility to be placed in proximity within an intracranial aneurysm. The central portion of the tubular member has a sufficiently decreased porosity to block blood flow from entering through the aneurysm. This is done by one of three methods: (1) the central portion of the member can be compressed to decrease porosity and heat set to hold the compression; (2) the angle of the fibers can be altered if the tubular member is made from a braided fibers; or (3) a monomeric coating can be formed on the central portion in an amount sufficient to decrease the porosity of the central portion upon polymerization of the monomeric coating. In the third embodiment a polymerization initiator is provided for polymerizing the monomeric coating upon command to cause the decreased porosity to block the blood flow. The device is heat set after compression to permit insertion and expansion in the patient. The tubular member has sufficient porosity at the two ends to keep open small perforator arteries proximate to the intracranial aneurysm.
Owner:GOBRAN RIAD H +2
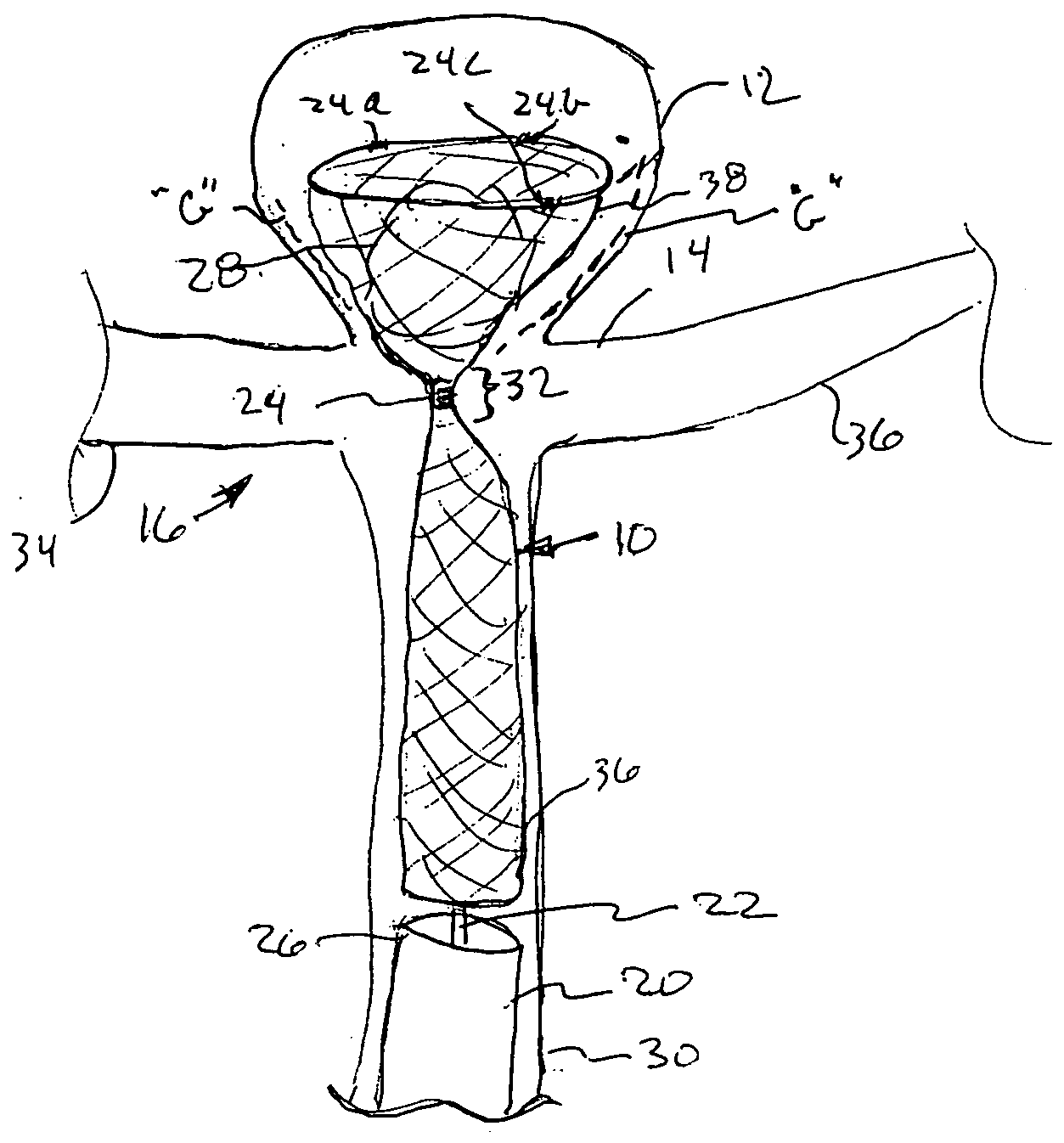
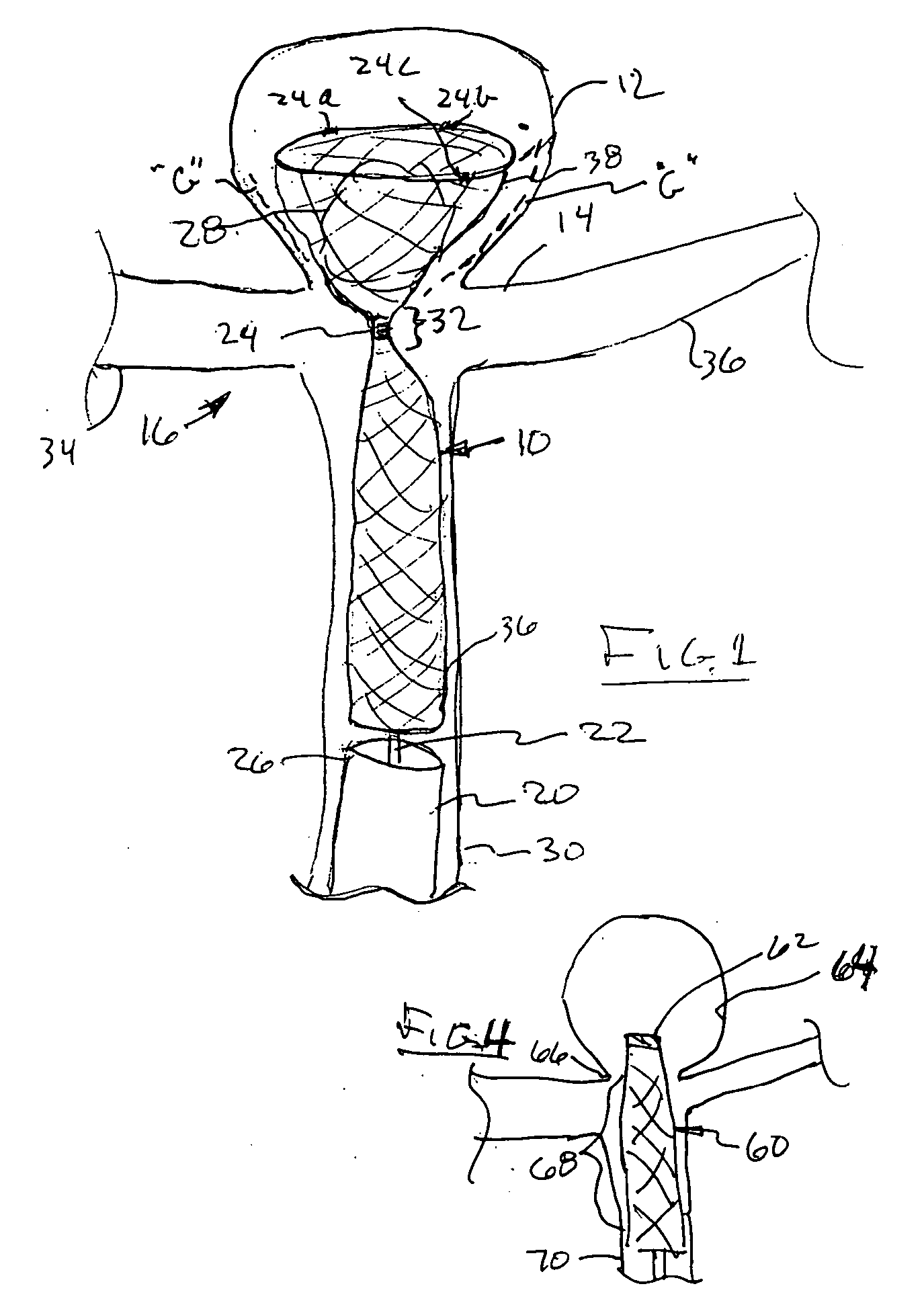
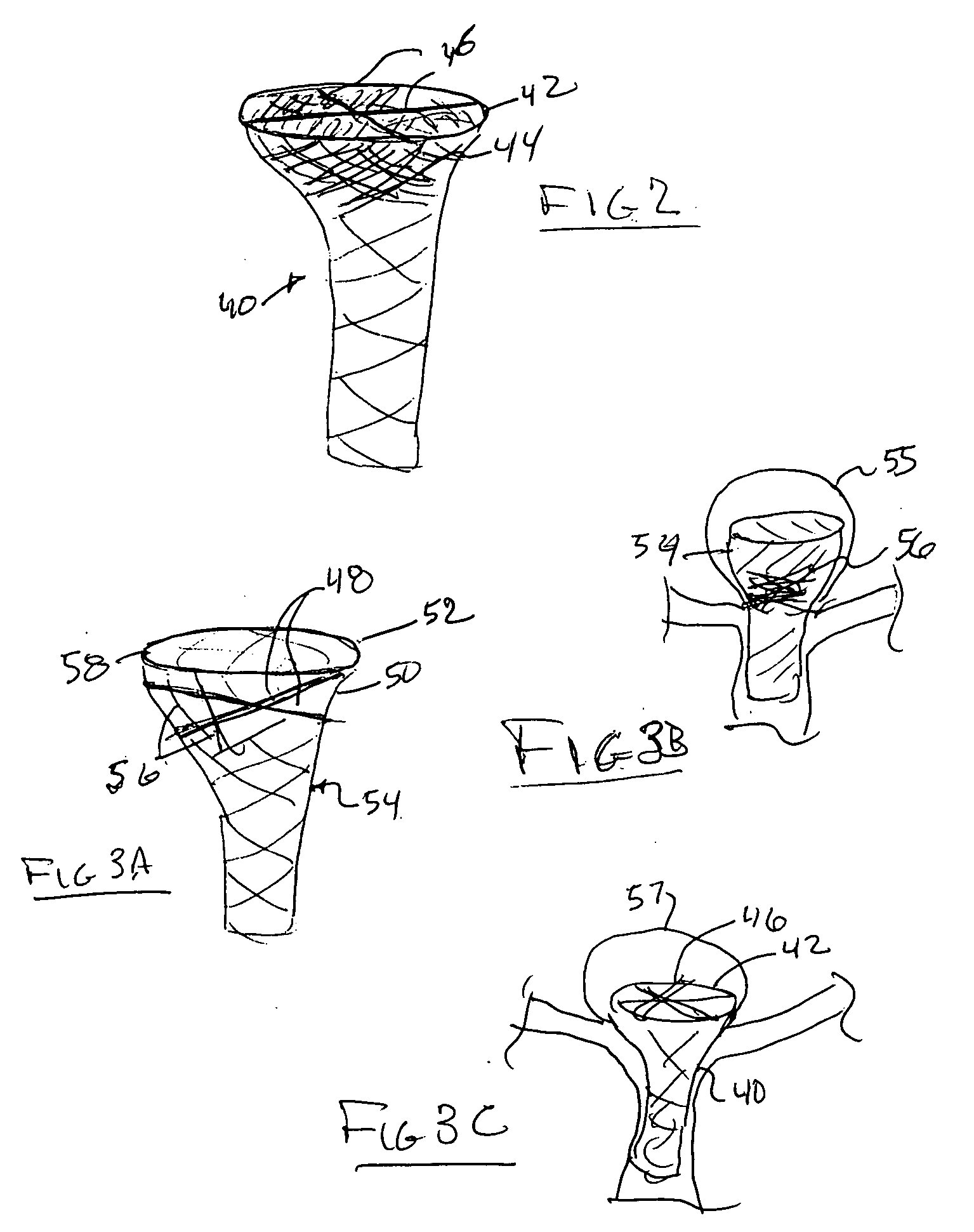
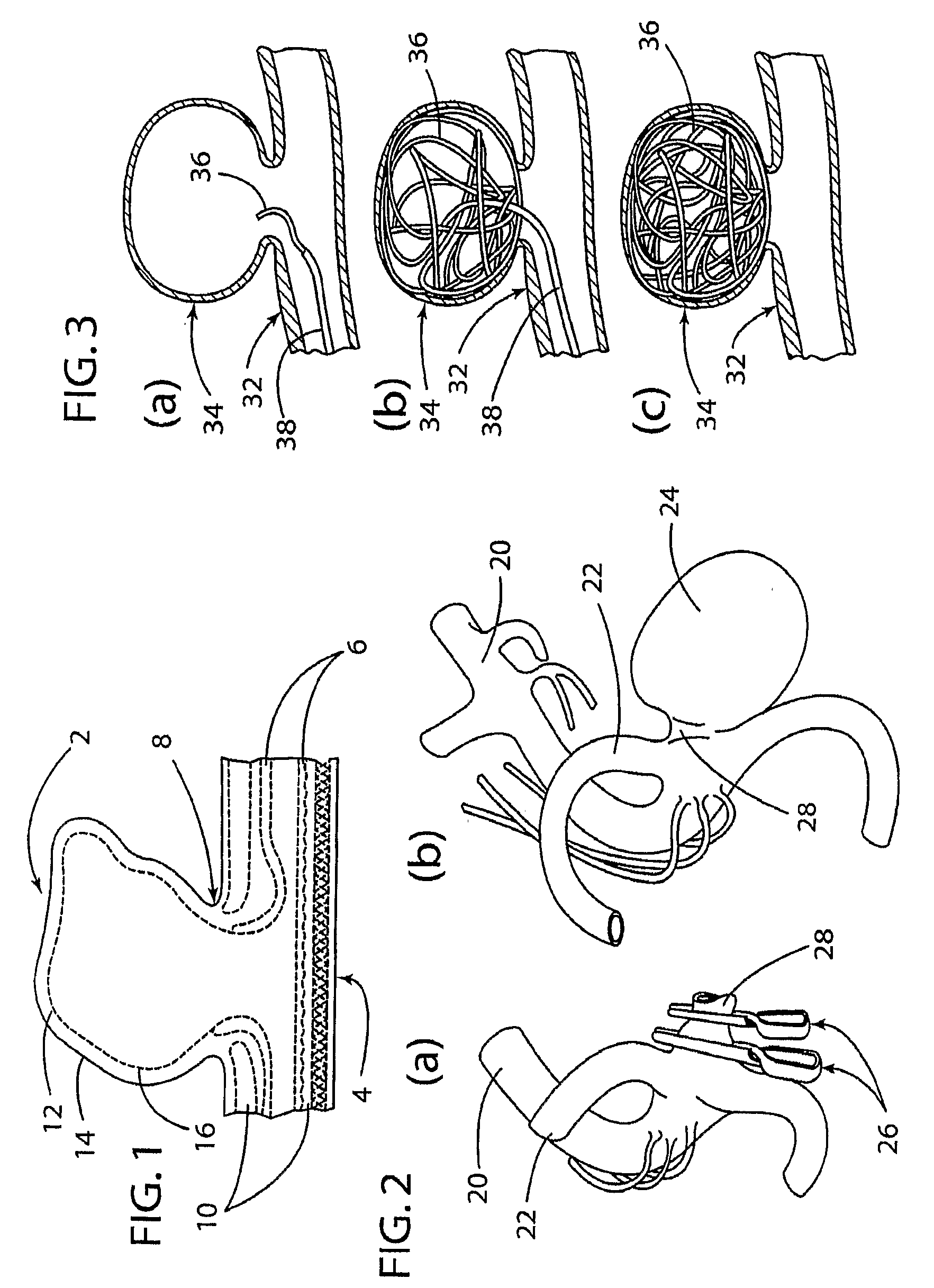
Aneurysm buttress arrangement
An aneurysm buttressing arrangement for covering an aneurysm opening in an intracranial aneurysm, for temporary placement thereadjacent, to prevent escape of embolitic agents from that aneurysm. The arrangement comprises an elongated delivery wire having a proximal end and a tracking distal end wire, a scaffold of expandable wires arranged proximal to and in spaced adjacent relationship to the distal end of the delivery wire, wherein the scaffold of wires has a tapered proximal end and a tapered distalmost end, the scaffold being expandable upon deployment from a delivery catheter, and collapsible for withdrawal into a delivery catheter, the tracking distal end wire extending distally from the scaffold about one-half to about ten centimeters.
Owner:NIAGARA GORGE MEDICAL DEVICES
Blood flow diverters for the treatment of intracranial aneurysms
InactiveUS20070060994A1Sufficient flexibility and body compatibilityReduce porosityBlood vesselsPorosityFiber
A blood flow diverter device for treatment of intracranial aneurysms, including a porous tubular member having a central portion and two ends. The member is of sufficient flexibility and body compatibility to be placed in proximity within an intracranial aneurysm. The central portion of the tubular member has a sufficiently decreased porosity to block blood flow from entering through the aneurysm. This is done by one of three methods: (1) the central portion of the member can be compressed to decrease porosity and heat set to hold the compression; (2) the angle of the fibers can be altered if the tubular member is made from a braided fibers; or (3) a monomeric coating can be formed on the central portion in an amount sufficient to decrease the porosity of the central portion upon polymerization of the monomeric coating. In the third embodiment a polymerization initiator is provided for polymerizing the monomeric coating upon command to cause the decreased porosity to block the blood flow. The device is heat set after compression to permit insertion and expansion in the patient. The tubular member has sufficient porosity at the two ends to keep open small perforator arteries proximate to the intracranial aneurysm.
Owner:GOBRAN RIAD H +2
Device and methods for non-surgical clipping of aneurysms
InactiveUS7601160B2Reduce riskReduce flowSuture equipmentsSurgical needlesAneurysm neckShape-memory alloy
The present invention relates to a device for the non-surgical clipping of aneurysms. The invention also includes methods of use to treat aneurysms, including intracranial aneurysms. The aneurysm is clipped by positioning a wire comprising a shape memory alloy, pre-set to a mutually twisted conformation, on opposite sides of the neck of the aneurysm and causing the wires to twist around each other. Thus, the aneurysm neck is substantially closed. The resulting thrombosis in the aneurysm further excludes the aneurysm from blood flow and pressure.
Owner:ZULI HLDG LTD
Bioabsorbable polymeric implants and a method of using the same to create occlusions
InactiveUS20020040239A1Peptide/protein ingredientsPharmaceutical containersPoly-L-lactideVascular compartment
A new embolic agent, bioabsorbable polymeric material (BPM) is incorporated to a Guglielmi detachable coil (GDC) to improve long-term anatomic results in the endovascular treatment of intracranial aneurysms. The embolic agent, comprised at least in part of at least one biocompatible and bioabsorbable polymer and growth factors, is carried by hybrid bioactive coils and is used to accelerate histopathologic transformation of unorganized clot into fibrous connective tissue in experimental aneurysms. An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location. Thrombogenicity of the biocompatible and bioabsorbable polymer is controlled by the composition of the polymer. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and bioabsorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid / poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides.
Owner:RGT UNIV OF CALIFORNIA
Aneurysm buttress arrangement
Owner:NIAGARA GORGE MEDICAL DEVICES
Photo-Activatable Gel Coated Intracranial Stent and Embolic Coil
ActiveUS20170266023A1Prevent premature activationAvoid contactStentsDiagnosticsMetallic materialsNeck of pancreas
An intracranial stent includes a proximal end, a distal end, and a tubular sidewall extending there between and a patch covering at least a portion of the sidewall; wherein the patch is capable of diverting blood flow past the neck of an intracranial aneurysm. The patch may be made of a photon-activatable material or a tightly woven metal material with a density greater than a density of the sidewall itself.
Owner:THOMAS JEFFREY E
Method for intracranial aneurysm analysis and endovascular intervention planning
ActiveUS20120323547A1High riskMedical simulationAnalogue computers for chemical processesEndovascular treatmentEndovascular interventions
A method (100) of aneurysm analysis (110) and virtual stent simulation (120) for endovascular treatment of sidewall intracranial aneurysms.
Owner:SIEMENS HEALTHCARE GMBH
Image simulation method for intracranial aneurysm interventional therapy stent implantation
InactiveCN103198202AFacilitated releaseGuaranteed continuous smoothnessSpecial data processing applicationsWeight adjustmentTomographic image
The invention provides a visualization calculation method for a whole intracranial aneurysm interventional therapy stent implantation process, establishes a stent release expansion model, provides an effective numerical simulation method for the intracranial aneurysm interventional therapy stent implantation, and provides a visualization method used for detection, calculation and analysis of the surgical planning of intracranial aneurysm interventional therapy. The adopted technical scheme is as follows: firstly, a blood vessel centerline is utilized to implant a numerical simulation stent into the blood vessel, and an active contour model is utilized to perform stent expansion, then the distance of each two nodes of the stent is changeless through optimization, and finally hemodynamics calculation and analysis are performed, and optimal configuration for stent implantation is simulated and calculated. The image simulation method can be directly applied to three-dimensional vascular angiography tomographic images, enables the stent to keep own geometric morphological characters through optimization, and can utilize weight adjustment to enable the stent to be clung to the blood vessel wall as far as possible. The simulation process can control the stent implantation position conveniently, and better clinical application value is achieved in the surgical planning of interventional therapy.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
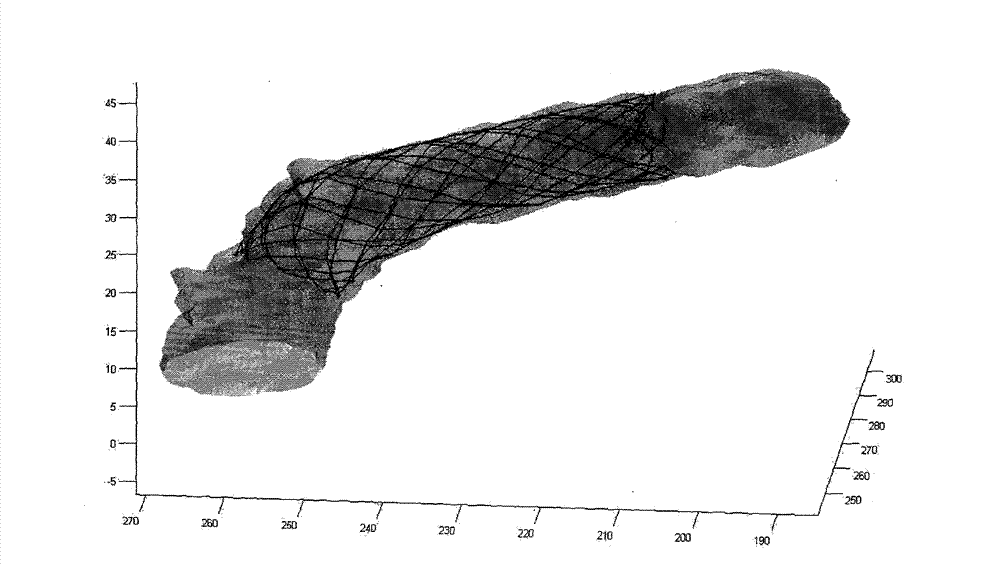
Intracranial aneurysm rupture risk assessment method and system
ActiveCN109907732AEffective treatment optionsImprove accuracyImage analysisHealth-index calculationAneurysm ruptureCvd risk
The invention discloses an intracranial aneurysm rupture risk assessment method and system. The method is applied to the intracranial aneurysm rupture risk assessment method and includes steps: creating a three-dimensional model comprising a parent artery and an aneurysm on the parent artery according to intracranial image data; determining target morphological parameters of a virtual parent artery and a virtual aneurysm on the basis of the three-dimensional model; determining target hemodynamic parameters of the virtual parent artery and the virtual aneurysm on the basis of the three-dimensional model; performing operation on the target morphological parameters, the target hemodynamic parameters and target clinical parameters on the basis of a pre-trained machine learning model to obtaina virtual aneurysm assessment result for assessing aneurysm rupture risks. Therefore, by implementation of the method and the system, accuracy in aneurysm rupture risk assessment can be improved through automatic comprehensive analysis of intracranial aneurysm rupture risks according to clinical parameters, morphological parameters and hemodynamic parameters of intracranial aneurysms of patients.
Owner:唯智医疗科技佛山有限公司
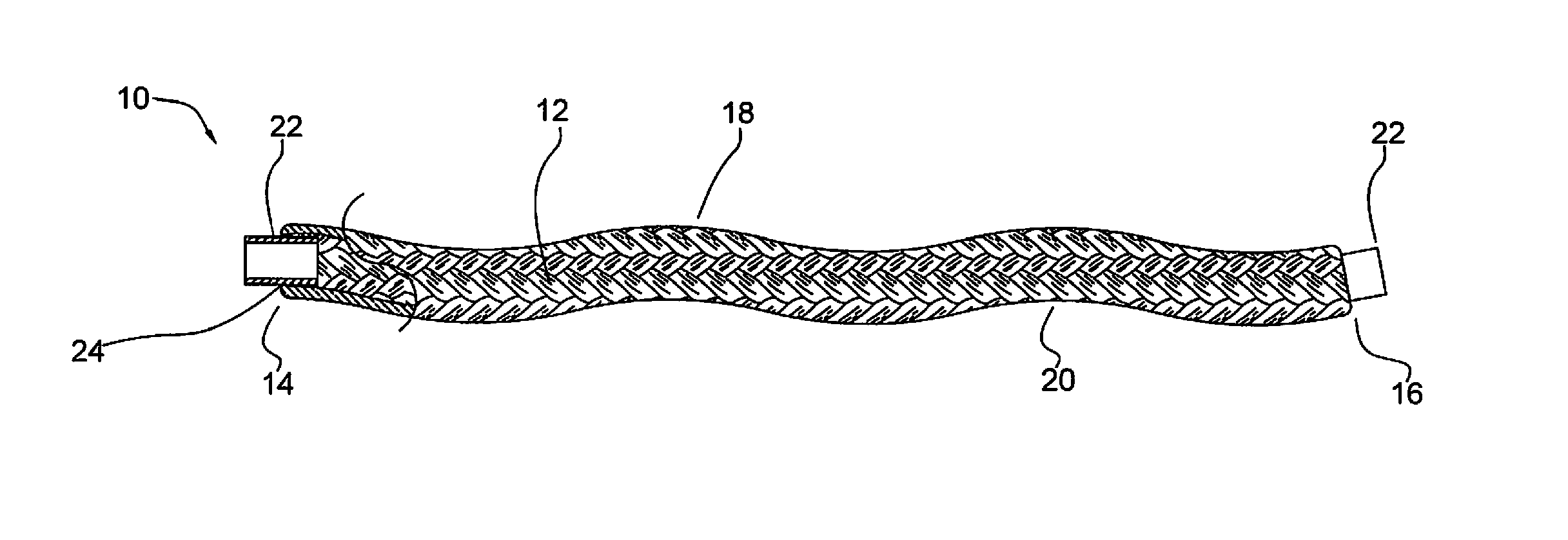
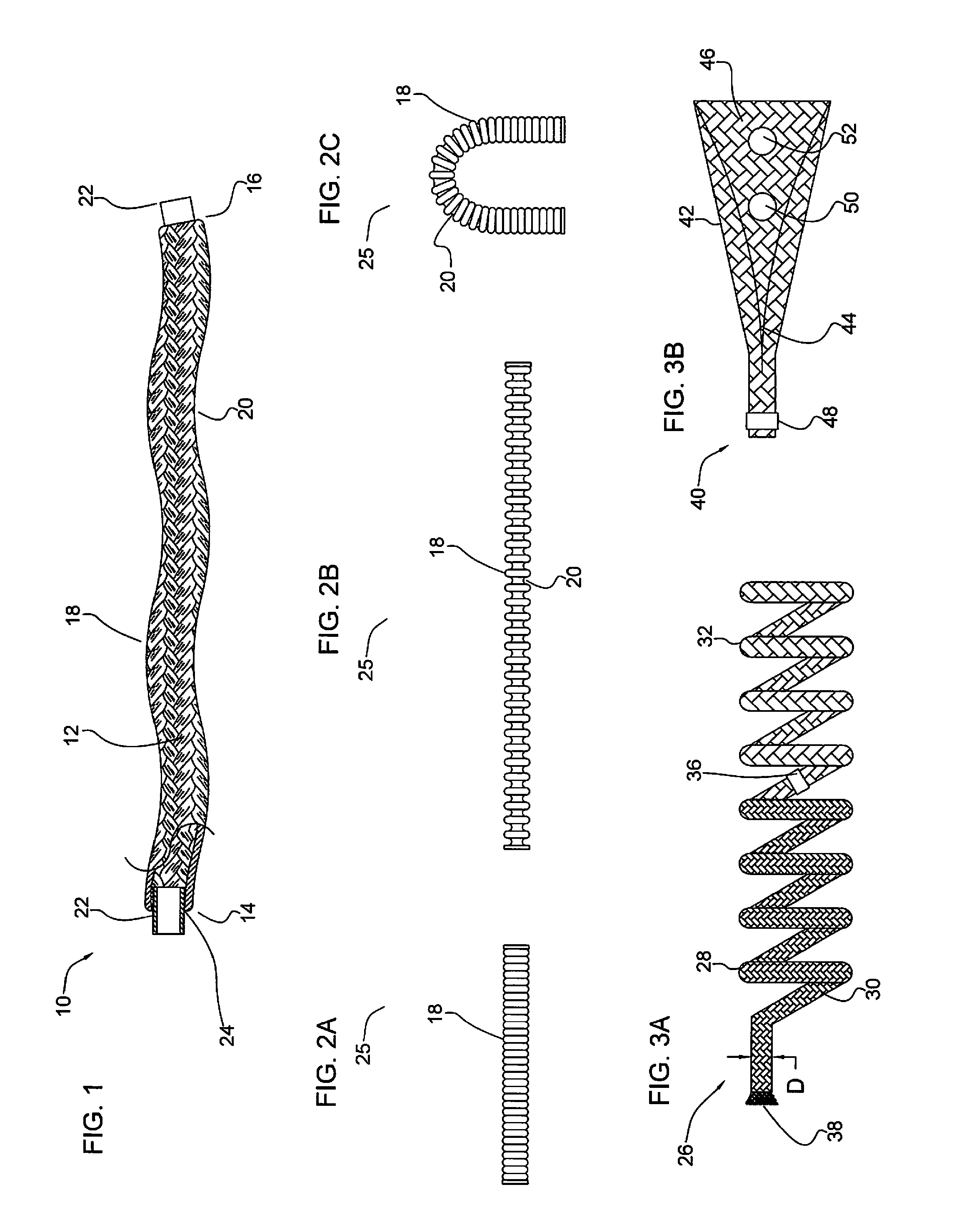
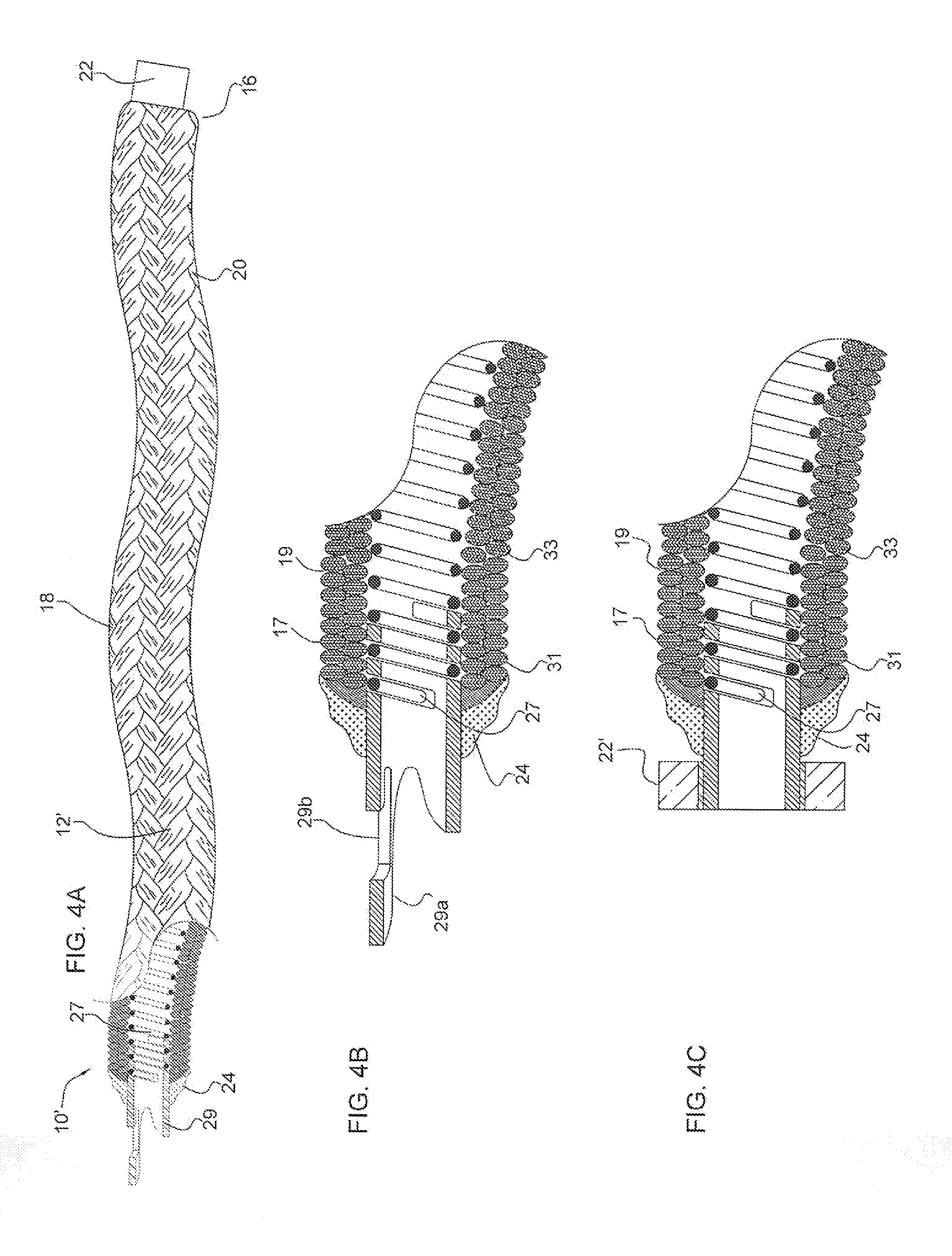
Micrograft for the treatment of intracranial aneurysms and method for use
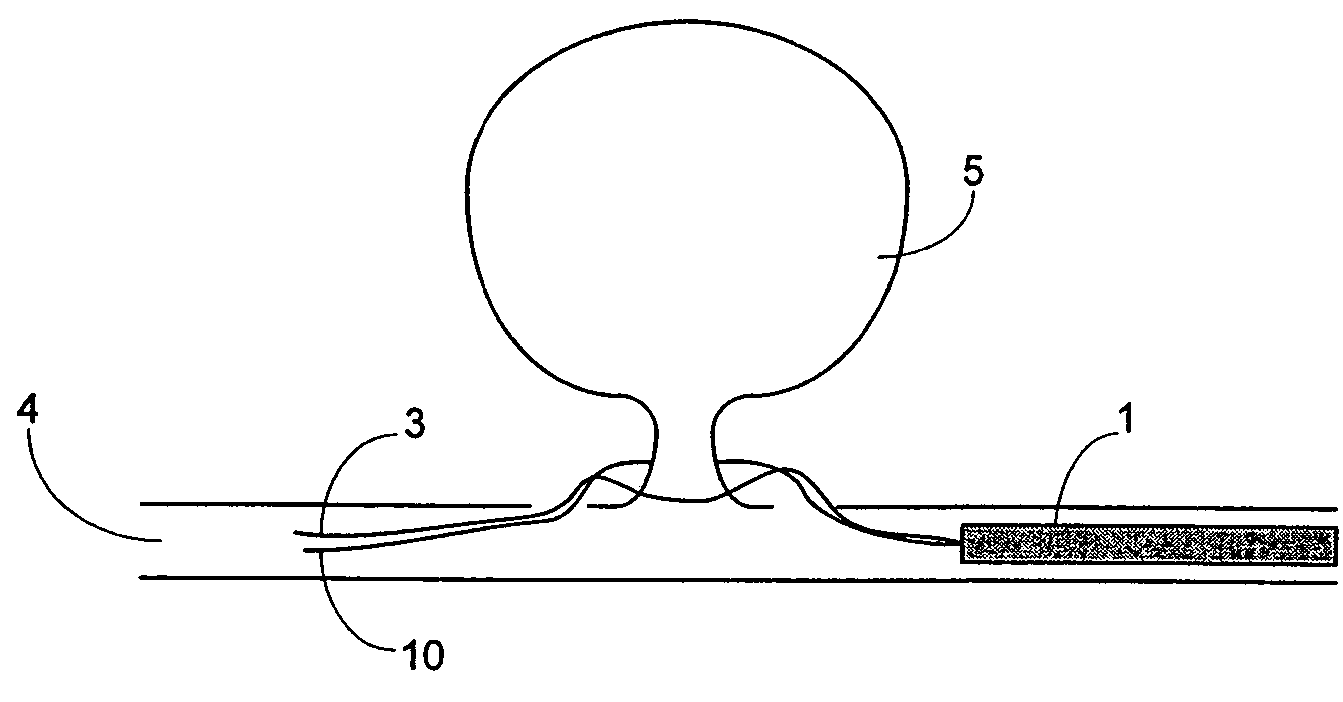
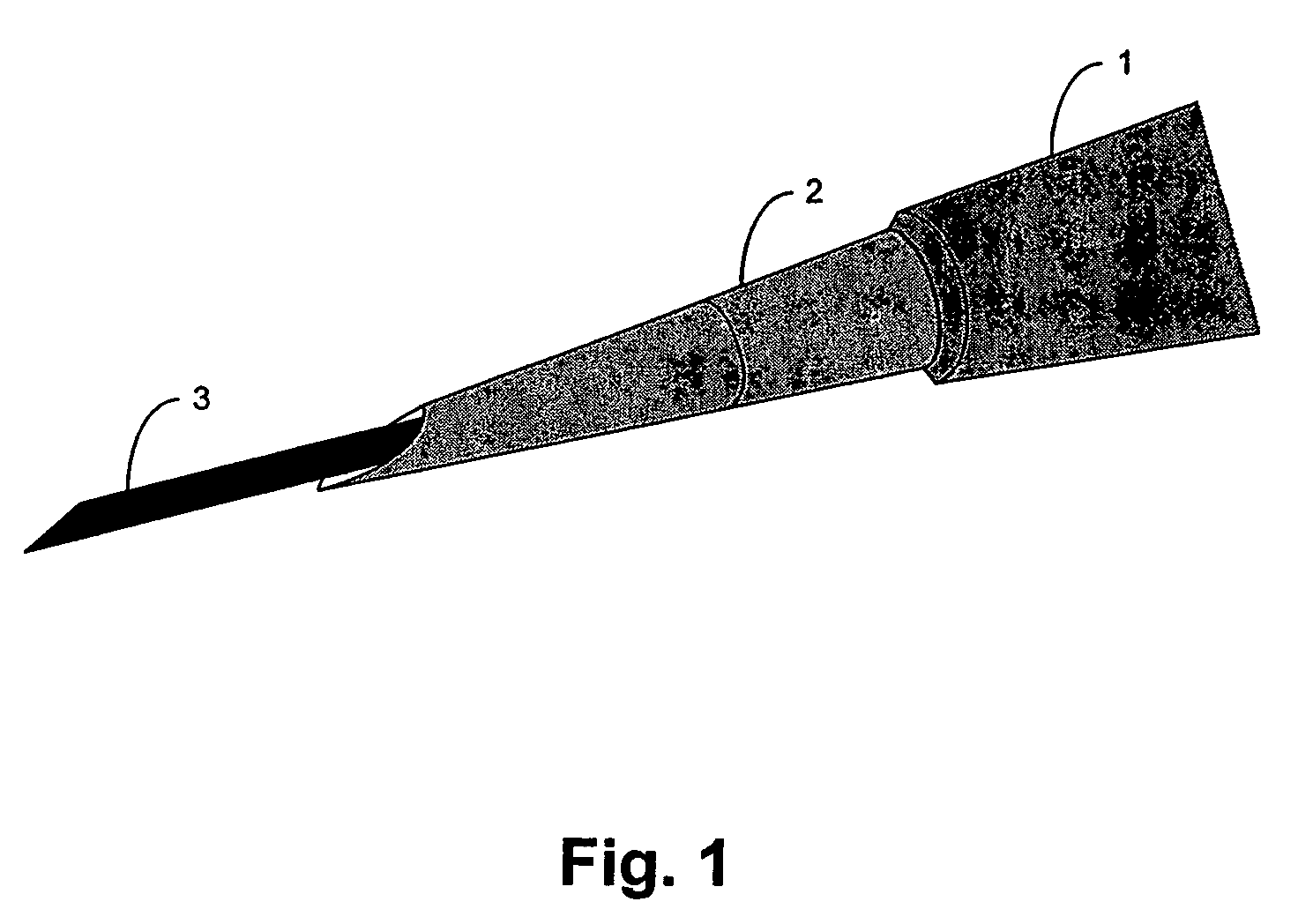
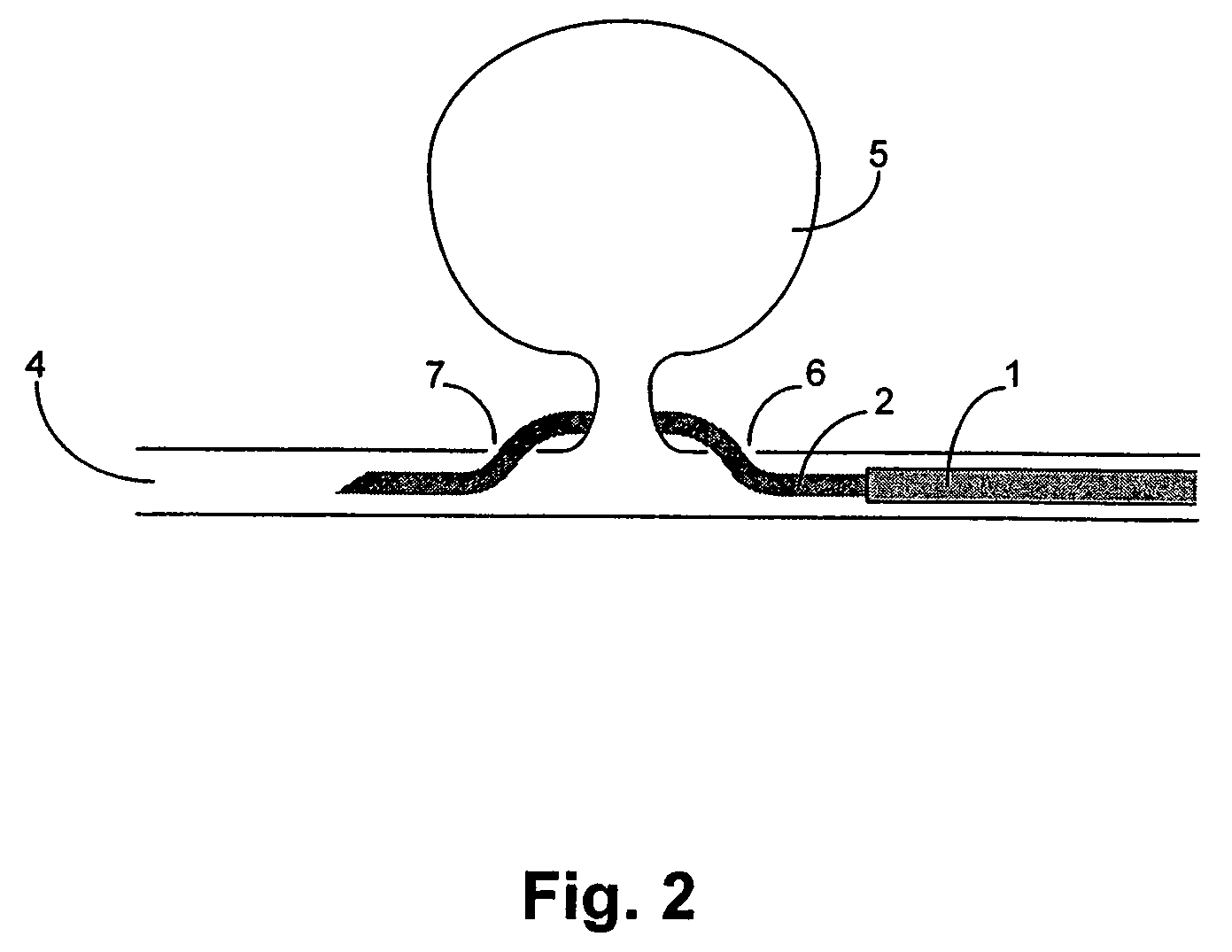
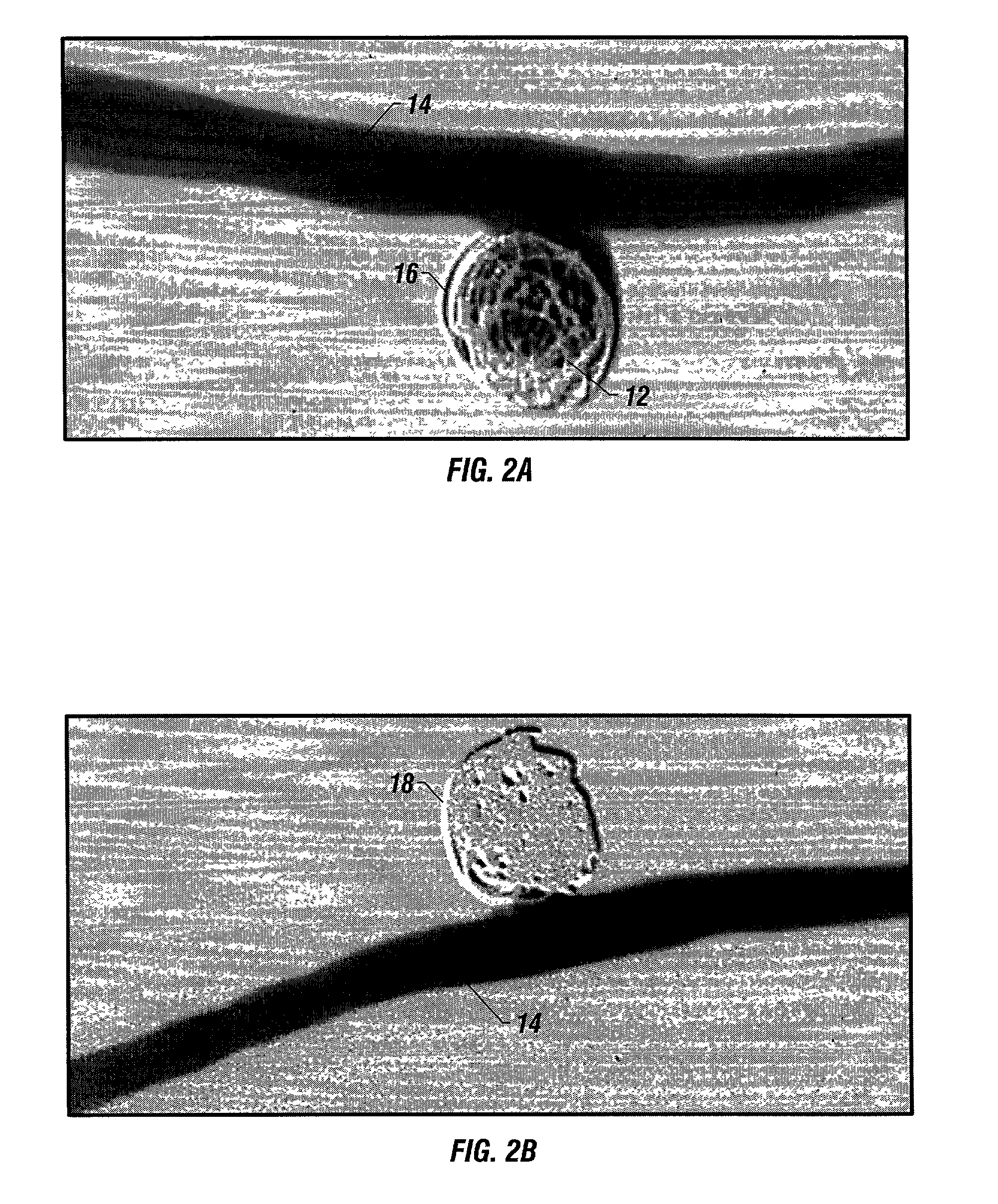
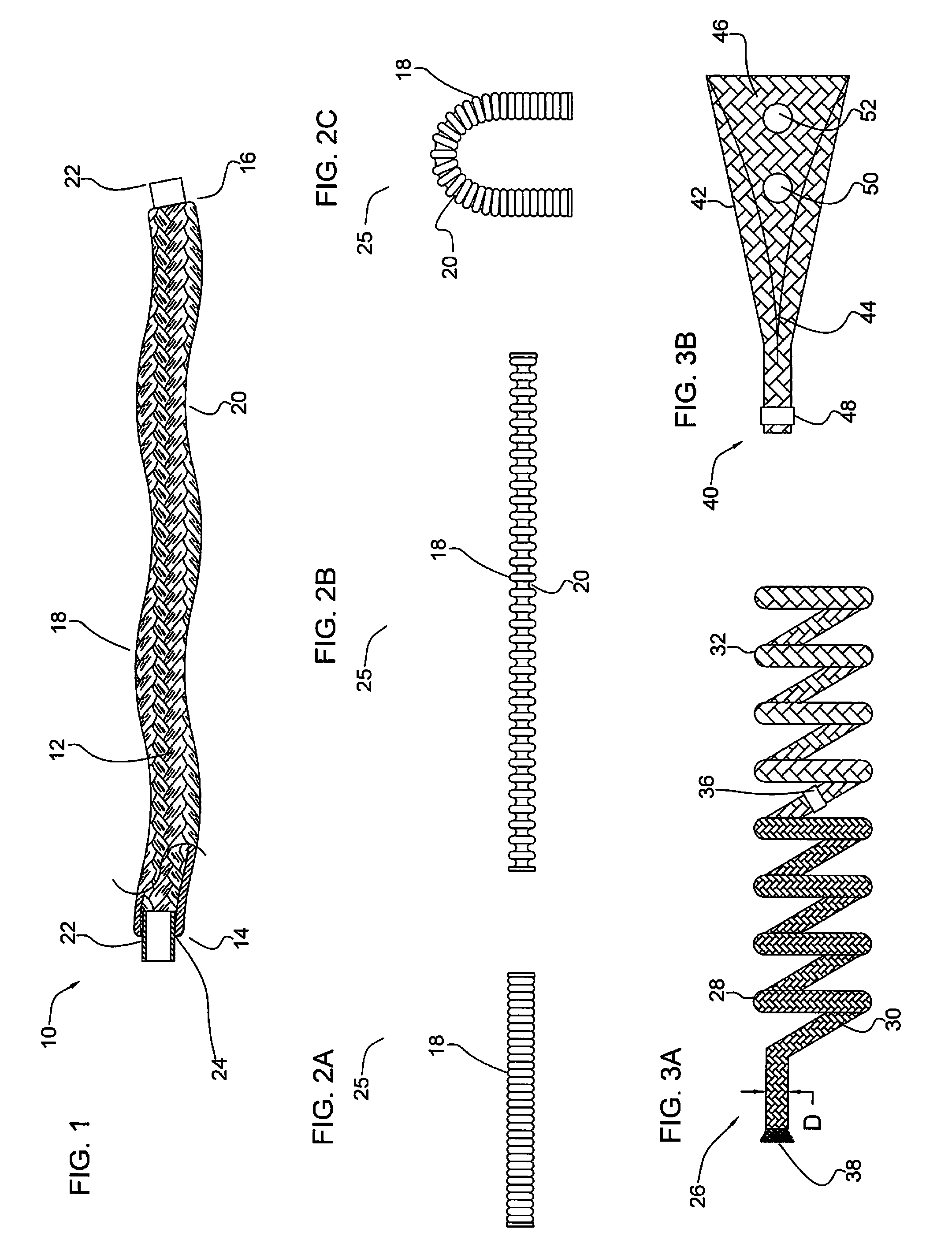
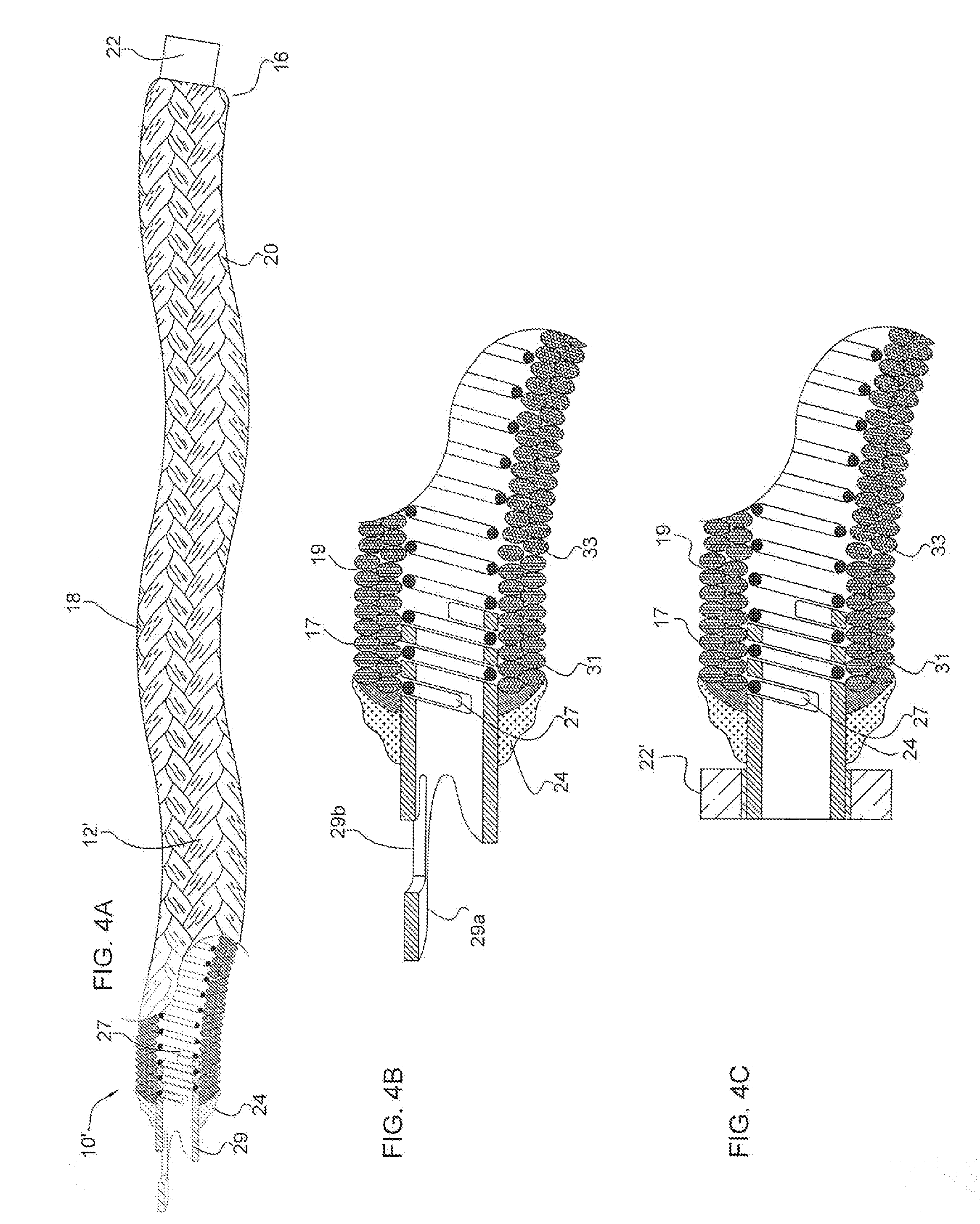
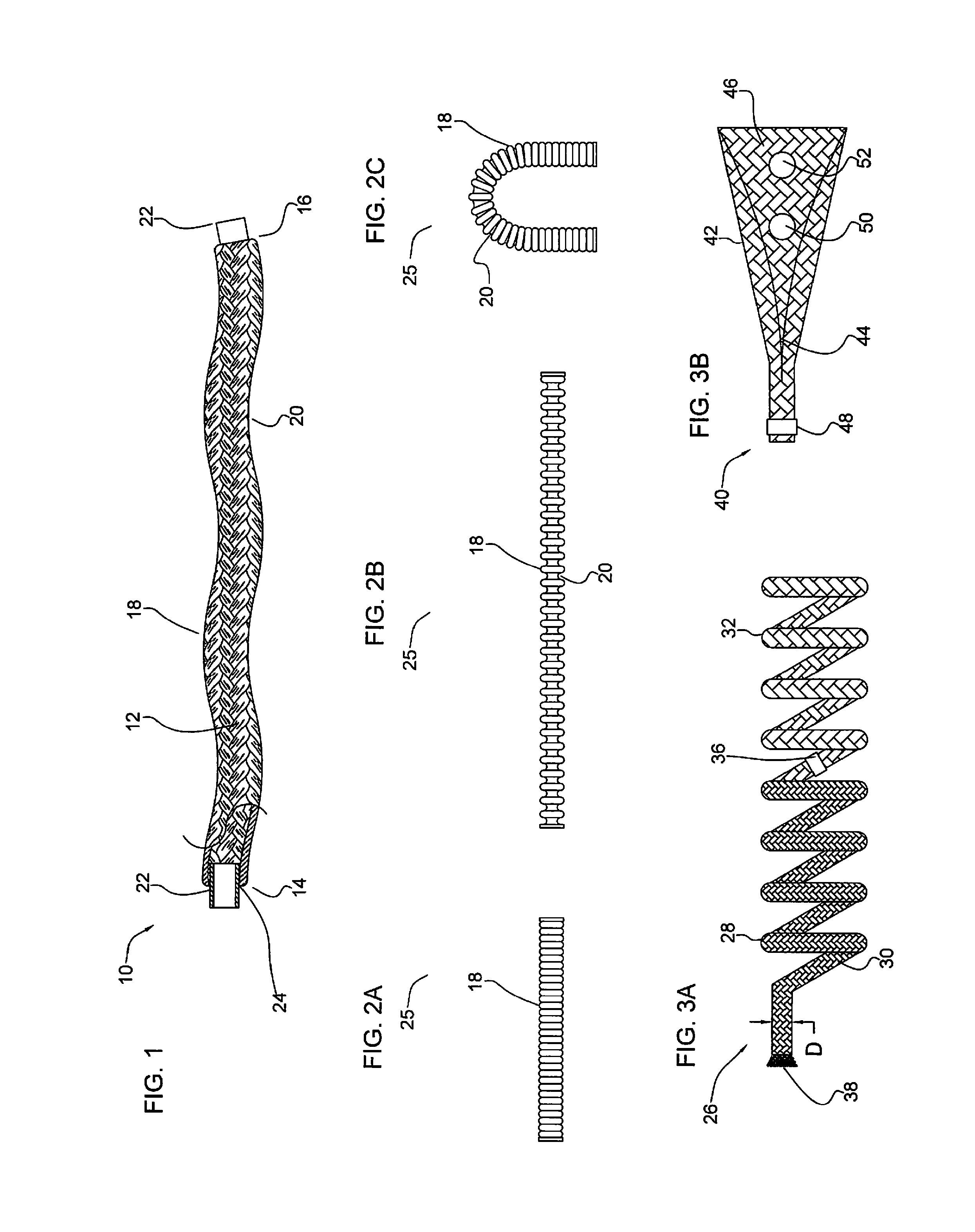
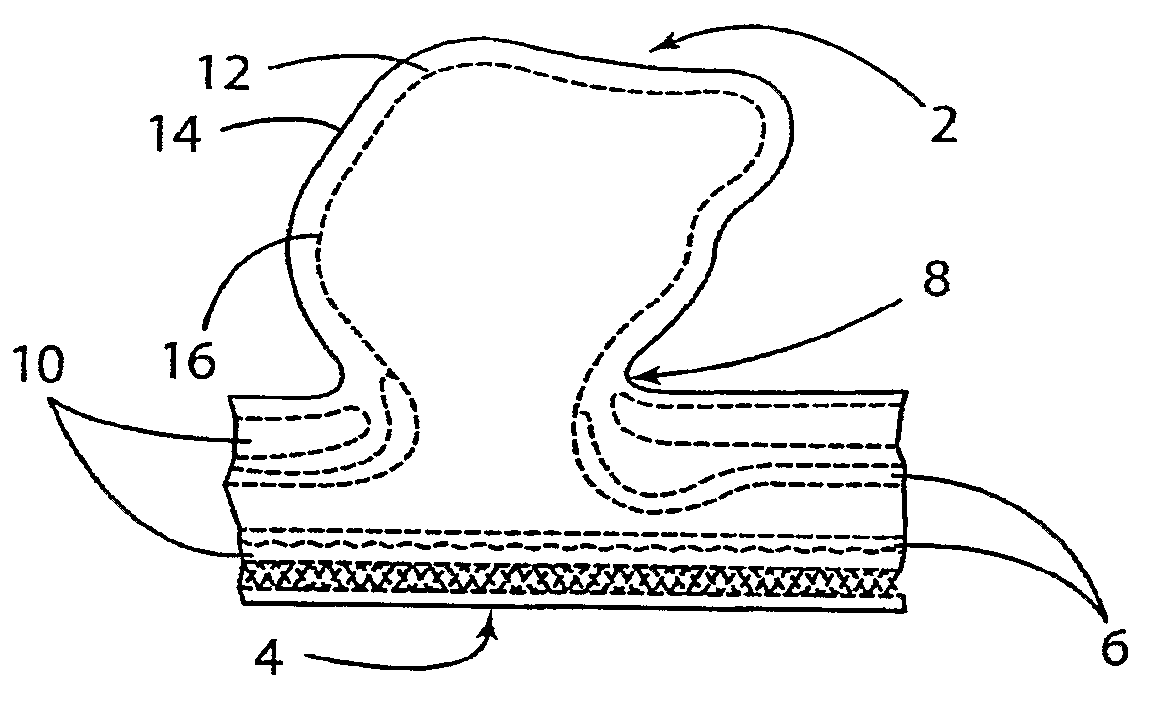
A device for occluding a vasculature of a patient including a micrograft having an absorbent polymeric structure with a lumen of transporting blood. The micrograft has a series of peaks and valleys formed by crimping. The occluding device is sufficiently small and flexible to be tracked on a guidewire and / or pushed through a microcatheter to a site within the vasculature of the patient. Delivery systems for delivering the micrografts are also disclosed.
Owner:NEUROGAMI MEDICAL INC
Micrograft for the treatment of intracranial aneurysms and method for use
A device for occluding a vasculature of a patient including a micrograft having an absorbent polymeric structure with a lumen of transporting blood. The micrograft has a series of peaks and valleys formed by crimping. The occluding device is sufficiently small and flexible to be tracked on a guidewire and / or pushed through a microcatheter to a site within the vasculature of the patient. Delivery systems for delivering the micrografts are also disclosed.
Owner:NEUROGAMI MEDICAL INC
Intracranial aneurysm interventional embolization treatment device
The invention discloses an intracranial aneurysm interventional embolization treatment device. The device comprises a plugging device and a conveying device. The plugging device is a net-shaped plugging object which is formed by knitting metal wires with a memory effect or high-elasticity polymer wires and developing wires. The plugging device comprises an upper part and a lower part which refer to an upper end intra-tumor part and a lower end extra-tumor part respectively. The upper end intra-tumor part and the lower end extra-tumor part are connected together to form a coating space for coating an aneurysmal neck. The conveying device comprises a conveying guide pipe and a conveying guide wire. In the conveying process, the plugging device is lengthened to be placed in the conveying guide pipe, and the conveying guide wire is connected with the tail of the plugging device. According to the intracranial aneurysm interventional embolization treatment device, intra-tumor thrombi can be induced to be formed, blood vessel repair of the aneurysmal neck is promoted, and therefore the purpose of healing arterial aneurysm is achieved. Moreover, parent blood vessels and normal branches cannot be affected obviously.
Owner:王奎重
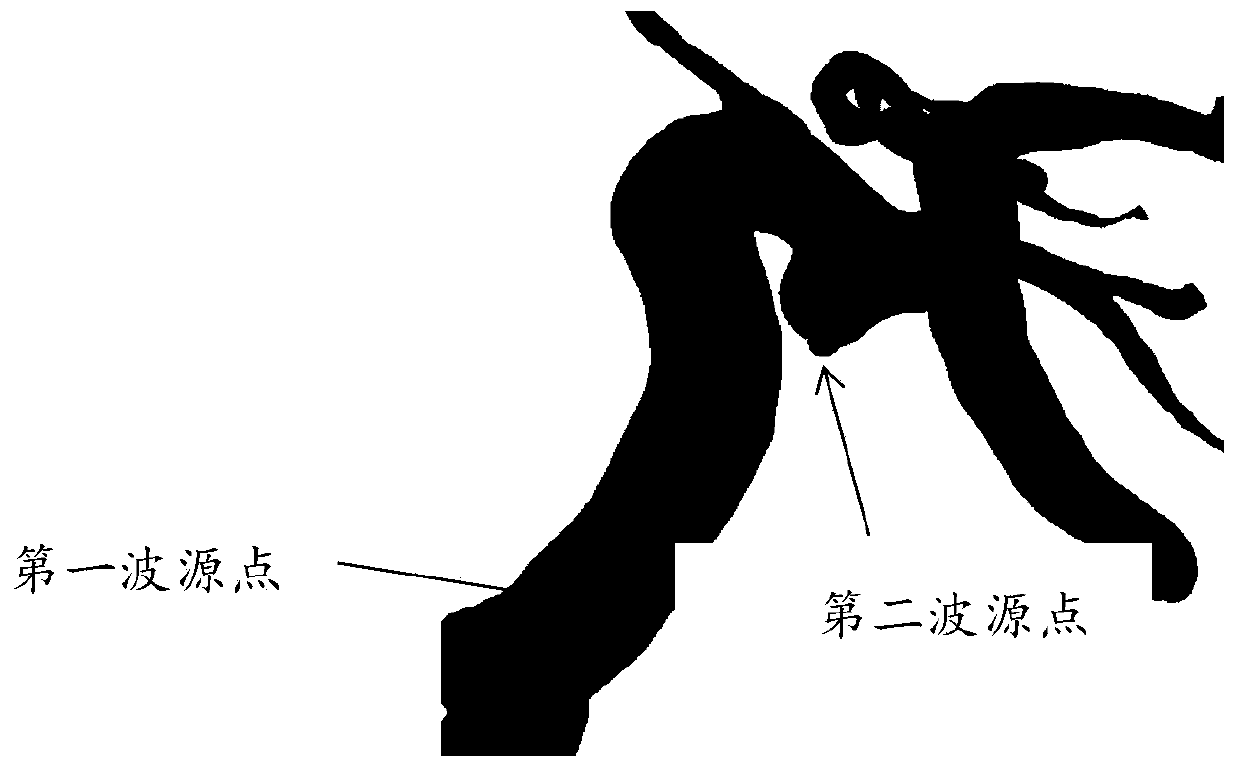
Method for aiding stent-assisted coiling of intracranial aneurysms by virtual parent artery reconstruction
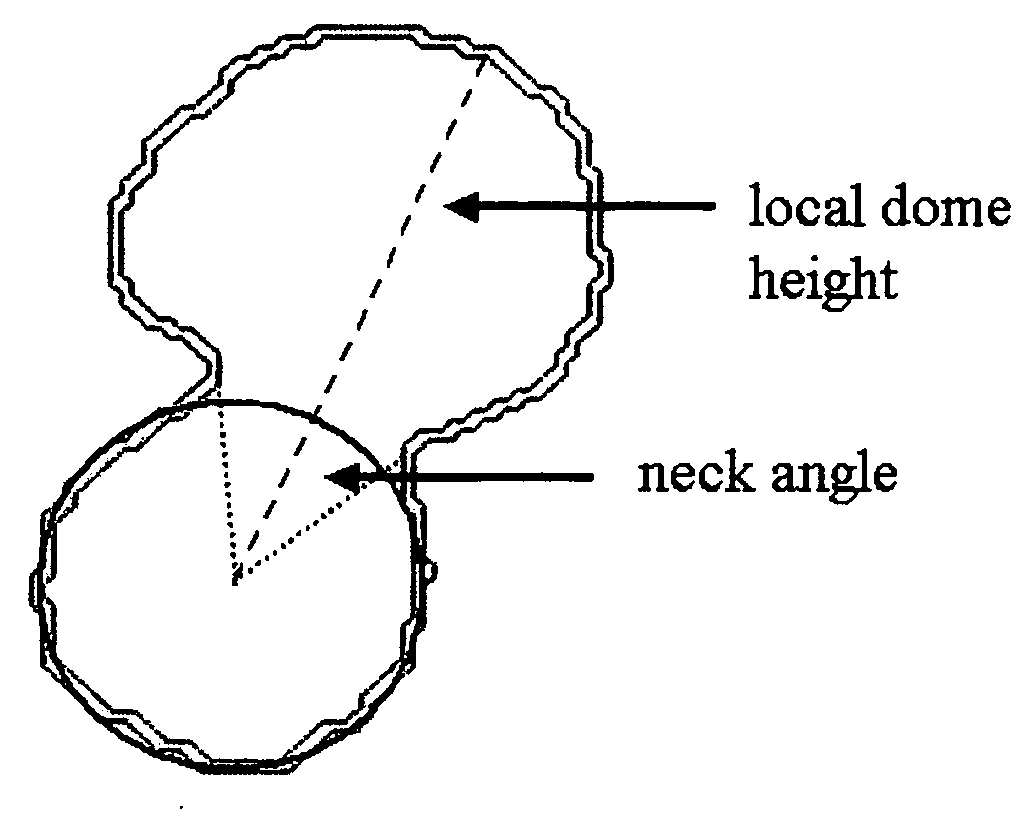
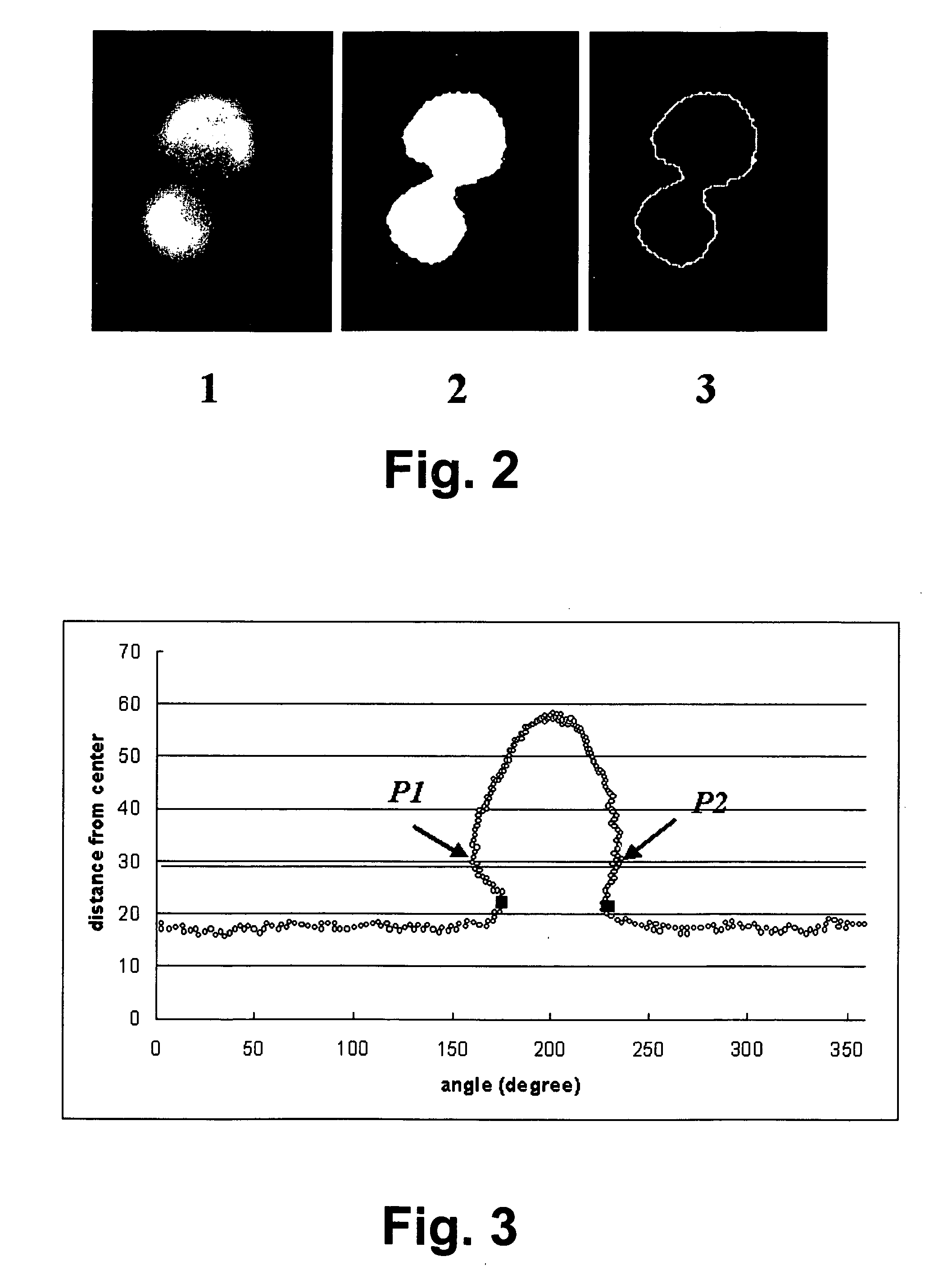
A method of creating a surface model of an intracranial aneurysm in an artery having a lumen, the aneurysm having a neck and a dome and the virtual reconstruction of the parent artery across the lateral extension of the aneurysm neck. The method includes the steps of: determining a center and radius of the artery over the lateral extension of the aneurysm; determining the boundary points that mark the boundary between the aneurysm neck and the artery; determining the angle of the aneurysm neck with respect to the artery, for various cross sections of the neck; determining the length of the neck; determining the height of the dome; estimating the area of the neck; and creating the surface model of the intracranial aneurysm in the artery, using the results from the previous steps.
Owner:BAYLOR COLLEGE OF MEDICINE
Method for non-invasive detection and treatment of cerebral aneurysms
ActiveUS20090068097A1Ultrasonic/sonic/infrasonic diagnosticsPeptide/protein ingredientsAntigenTherapeutic intent
Described herein is a method for non-invasive detection and treatment of intra-cranial aneurysms. Antibodies are provided to specifically react / bind with antigens of the cerebral aneurism wall. The antibodies may be bound to a label and / or to a therapeutic agent for diagnosis and / or for treatment purposes thereof. Intra-cranial aneurysms are thus non-invasively detected before rupture occurs and are specifically treated.
Owner:LERS SURGICAL
Genetic susceptibility variants associated with cardiovascular disease
ActiveUS20100068705A1Increased susceptibilityReduce sensitivityMicrobiological testing/measurementBiological testingCoronary artery diseaseCoronary heart disease
The invention relates to methods of diagnosing susceptibility to cardiovascular disease, including coronary artery disease. MI, abdominal aorta aneurysm, intracranial aneurysm restenosis and peripheral arterial disease, by assessing the presence or absence of alleles of certain polymorphic markers found to be associates with cardiovascular disease. The invention further relates to kits encompassing reagents for assessing such markers, and methods for assessing the probability of response to therapeutic agents and methods using such markers.
Owner:DECODE GENETICS EHF
Method for evaluating intracranial aneurysm rupture risk, device and computer equipment
PendingCN109961850ASolving a major challenge in fracture risk assessmentThe conclusion is accurateDiagnostic signal processingHealth-index calculationAneurysm ruptureIliac Aneurysm
The invention relates to a method for evaluating an intracranial aneurysm rupture risk. The method comprises the steps of acquiring image data; acquiring a starting point and a terminal point in a to-be-evaluated blood vessel for obtaining a segment of a target blood vessel; cutting the target blood vessel through a cutting plane for segmenting an aneurysm wall and a vessel wall; performing analysis and acquiring parameter information of the aneurysm and the blood vessel around the aneurysm; distributing the blood flow amount according to the parameter information of the aneurysm and the bloodvessel, and performing blood flow simulation on the blood vessel and the aneurysm through a volume simulating algorithm, and extracting multiple wall surface shearing stress parameters of the aneurysm and the corresponding blood flow dynamic parameter according to a simulating result; acquiring multiple kinds of data of clinical parameters; and performing calculation on the clinical parameter, the multiple wall surface shearing stress parameters of the aneurysm, the corresponding blood flow dynamic parameter and the parameter information of the aneurysm and the blood vessel which bears the aneurysm according to a machine learning algorithm for calculating the aneurysm rupture risk.
Owner:广州新脉科技有限公司 +1
Bioabsorbable polymeric implants and a method of using the same to create occlusions
A new embolic agent, bioabsorbable polymeric material (BPM) is incorporated to a Guglielmi detachable coil (GDC) to improve long-term anatomic results in the endovascular treatment of intracranial aneurysms. The embolic agent, comprised at least in part of at least one biocompatible and bioabsorbable polymer and growth factors, is carried by hybrid bioactive coils and is used to accelerate histopathologic transformation of unorganized clot into fibrous connective tissue in experimental aneurysms. An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location. Thrombogenicity of the biocompatible and bioabsorbable polymer is controlled by the composition of the polymer. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and bioabsorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly˜glycolic acid / poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides.
Owner:RGT UNIV OF CALIFORNIA
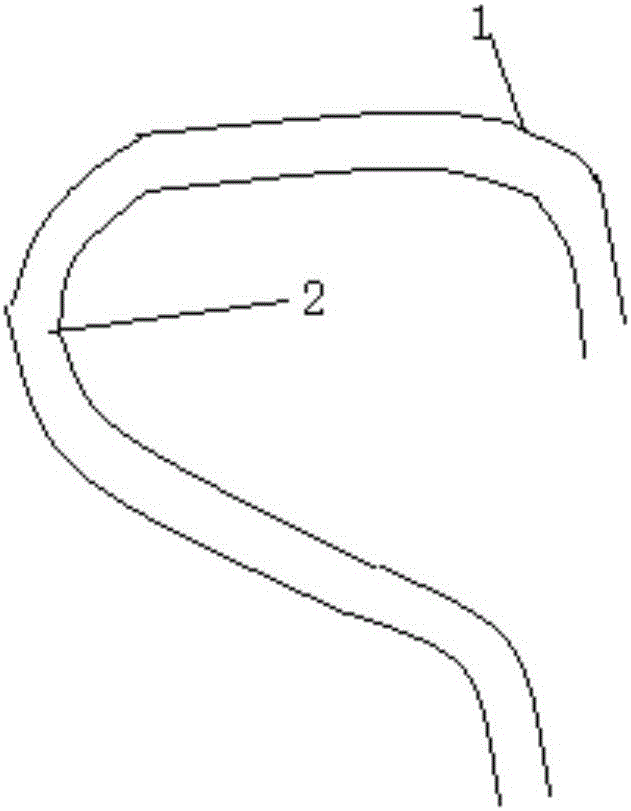
Microcatheter moulding stent used for intracranial aneurysm embolization and preparation method of microcatheter moulding stent
InactiveCN106037853AAvoid disadvantagesSave operating timeComputer-aided planning/modellingOcculdersInsertion stentIliac Aneurysm
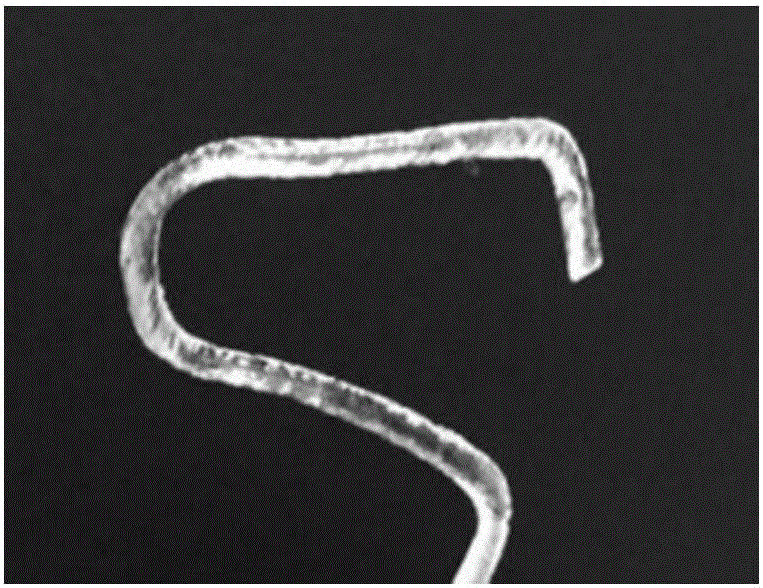
The invention relates to a microcatheter moulding stent used for the intracranial aneurysm embolization and a preparation method of the microcatheter moulding stent. The microcatheter moulding stent comprises a first bending part and a second bending part, wherein the first bending part is connected with the second bending part, the length of the first bending part is 3-5 mm, the length of the second bending part is 5-15 mm, the diameter of the stent is 1-1.5 mm, the length of the stent is 30-50 mm, and the first bending part and the second bending part are used for closely fitting the intracranial vascular bending and arterial aneurysm positions. According to the microcatheter moulding stent and the preparation method, the bending degree of the stent closely fits the intracranial vascular bending and arterial aneurysm shapes, so that the accurate moulding of the microcatheter is guided, therefore, the accurate placement of the microcatheter in the surgery is facilitated, and further, the operative problems including the difficulty in placement of the microcatheter, the separation of the microcatheter, the floating of a spring ring, and the like caused by poor moulding of the microcatheter in the embolism process are avoided.
Owner:李泽福
Intracranial aneurism rupture risk assessment and image classification apparatus
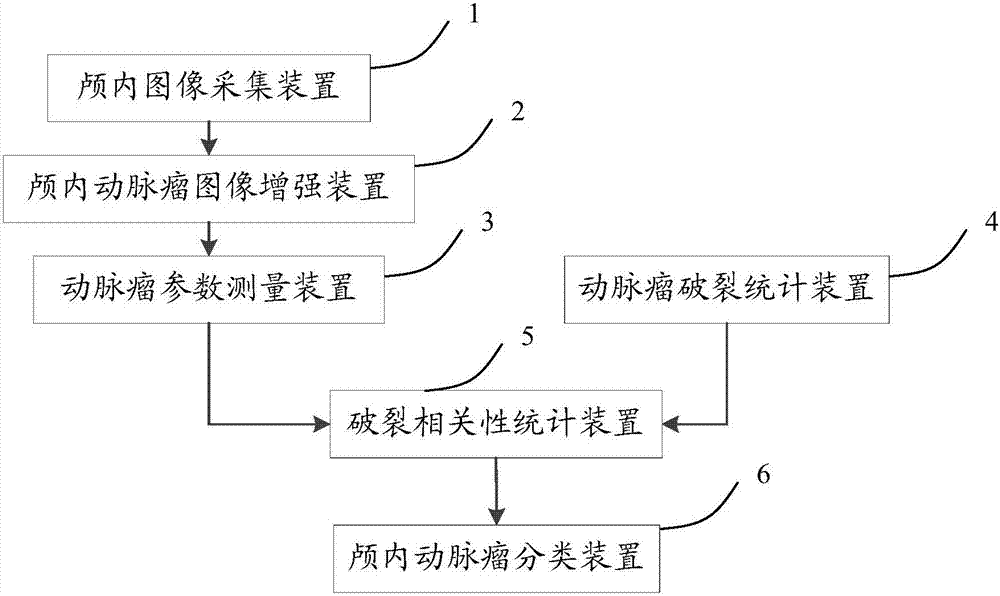
ActiveCN107273658AOutstanding FeaturesOutstanding mechanicsImage enhancementImage analysisCvd riskRupture risk
The invention discloses an intracranial aneurism rupture risk assessment and image classification apparatus. The apparatus comprises an intracranial image collection apparatus used for collecting intracranial images, an intracranial aneurism image enhancement apparatus used for performing image enhancement on the collected intracranial images, confirming the intracranial images in which intracranial aneurisms exist and processing the intracranial images to obtain enhanced intracranial aneurism images, an aneurism parameter measurement apparatus used for performing measurement and calculation on the enhanced intracranial aneurism images in a stereoscopic display environment to obtain specific numerical values of a plurality of morphologic parameters, an aneurism rupture statistics apparatus used for performing statistics on the enhanced intracranial aneurism images in a pathological database to obtain ruptured cases and unruptured cases in the images, a rupture relevance statistics apparatus used for obtaining a rupture relevance statistics result, and an intracranial aneurism classification apparatus used for classifying new cases according to the rupture relevance statistics result and outputting a classification result.
Owner:HARBIN MEDICAL UNIVERSITY
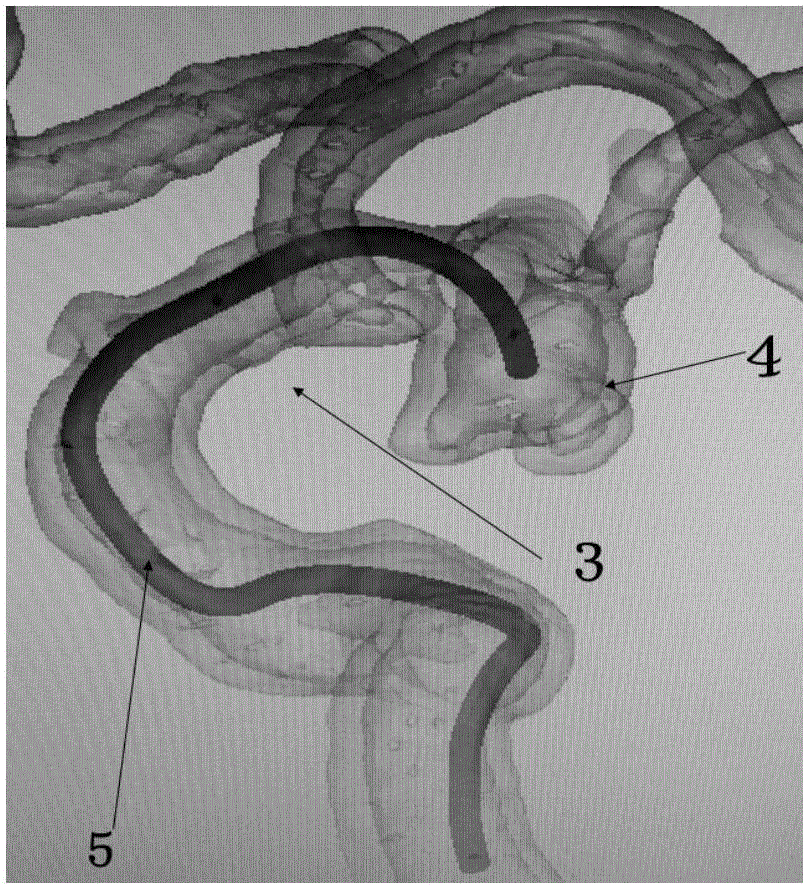
Simulation method for intracranial aneurysm treatment and control device
ActiveCN107049487ACalculation method is simpleImprove simulation speedComputer-aided planning/modellingSpecial data processing applicationsInsertion stentEuler bernoulli beam
The invention provides a simulation method and device for intracranial aneurysm treatment. The method comprises the following steps: establishing a three-dimensional structure of a spring ring and placing the spring ring into an arterial aneurysm: packaging the spring ring into a simulated catheter in a simulated calculation manner and then releasing the spring ring into the arterial aneurysm; establishing a three-dimensional structure of a stent and placing the stent into an arterial aneurysm carrying blood vessel: compressing and placing the stent into a micro catheter in a simulated calculation manner, then bending the stent and the micro catheter, and releasing the stent into the arterial aneurysm carrying blood vessel; establishing a structure of the spring ring, and when the spring ring is placed in the arterial aneurysm, simulating the spring ring into a three-dimensional Euler-Bernoulli beam; establishing a three-dimensional structure of the stent, and when the stent is placed in the arterial aneurysm carrying blood vessel, simulating the stent into a three-dimensional shell unit; when the stent is released into the arterial aneurysm carrying blood vessel, bending the stent and the micro catheter along the central line of the arterial aneurysm carrying blood vessel. The device is used for implementing the simulation method. According to the simulation method and the device, the simulation calculation efficiency can be improved, and the calculation time is shortened.
Owner:HANGZHOU ARTERYFLOW TECH CO LTD
Self-expandable segment tectorial asymmetric areolate stent
The invention provides a self-expandable segment tectorial asymmetric areolate stent for intracranial aneurysm. In the stent, a tubular hollow structure frame consists of two spirally and symmetrically arranged main support rods and asymmetrically arranged V-shaped connecting rings, and an externally applied membrane material is coated on the frame to form the stent. The releasing mode of the stent is self-expandable release, so that the stent is convenient to convey by virtue of a microcatheter system, is good in flexible performance, and is especially suitable for being applied to intracranial curved section vascular aneurismal lesion.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Coating film capable of adhering or directly adhering on blood vessel
ActiveCN102824198AWith thermal shrinkage and cold expansionEasy to operateSurgeryCoatingsCarotid-cavernous fistulaEmbolization material
The invention provides a coating film capable of adhering or directly adhering on blood vessel. Surface of the inventive artificial blood vessel coating film is coated with a nano-gel coating layer made from mixture of adhesive embolic material and poly(N-isopropylacrylamide). After release of the adhesive embolic material in the nano-gel coating layer, the artificial blood vessel coating film is tightly bonded with blood vessel intima, and simultaneously blocks aneurysm to isolate aneurysm from blood flow. The inventive coating film has the advantages of simple operation during surgery, low cost, and high safety and reliability; is suitable for pathology such as intracranial aneurysms, carotid cavernous fistula (CCF), arteriovenous fistula, thoracic aortic aneurysm and abdominal aortic aneurysm; and has wide application in medical field.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Intracranial aneurysm interventional closure treatment device
The invention relates to an intracranial aneurysm interventional closure treatment device. The intracranial aneurysm interventional closure treatment device includes a closure device, a delivery device, and a closure device fixing frame; the closure device fixing frame is circular tube-shaped, and the wall of the circular tube is of a multi-hole net structure; the closure device includes a closure device outside-aneurysm part, a closure device fixing part, and a connection part connecting the closure device outside-aneurysm part and the closure device fixing part; the closure device outside-aneurysm part is a disc-shaped closure piece and tightly attaches to the inner wall of a support or the inner wall of a blood vessel; the closure device fixing part is a circular table-shaped closure piece which tightly attaches to the closure device fixing frame; and a far end of the closure device outside-aneurysm part contracts inward and is connected to the closure device fixing part. During closure, the connection part of the closure device is embedded into net holes of the closure device fixing frame, the closure device fixing part is positioned outside the wall of the closure device fixing frame, and the closure device outside-aneurysm part is positioned inside the wall of the closure device fixing frame. The closure treatment device can achieve complete cover of an aneurysm neck, induce thrombosis in the aneurysm, and achieve complete embolism of the aneurysm.
Owner:张小曦
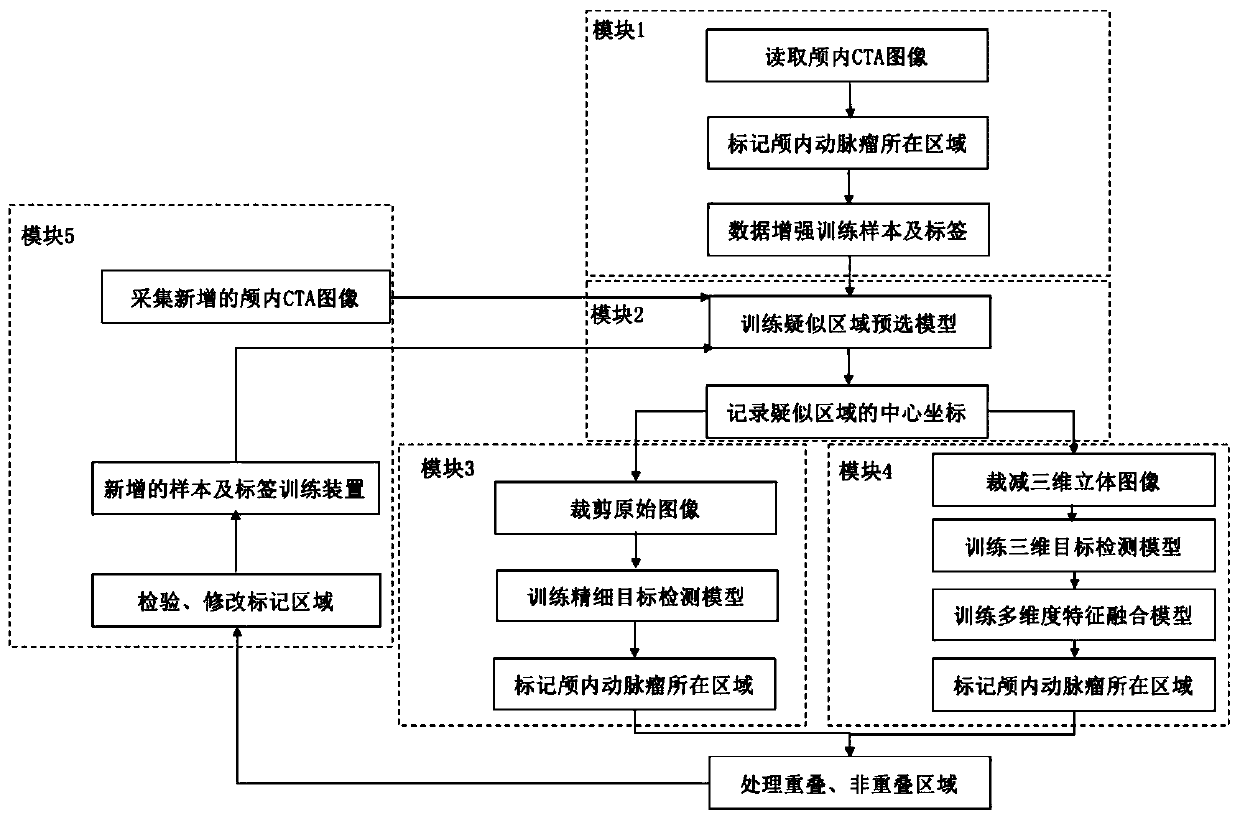
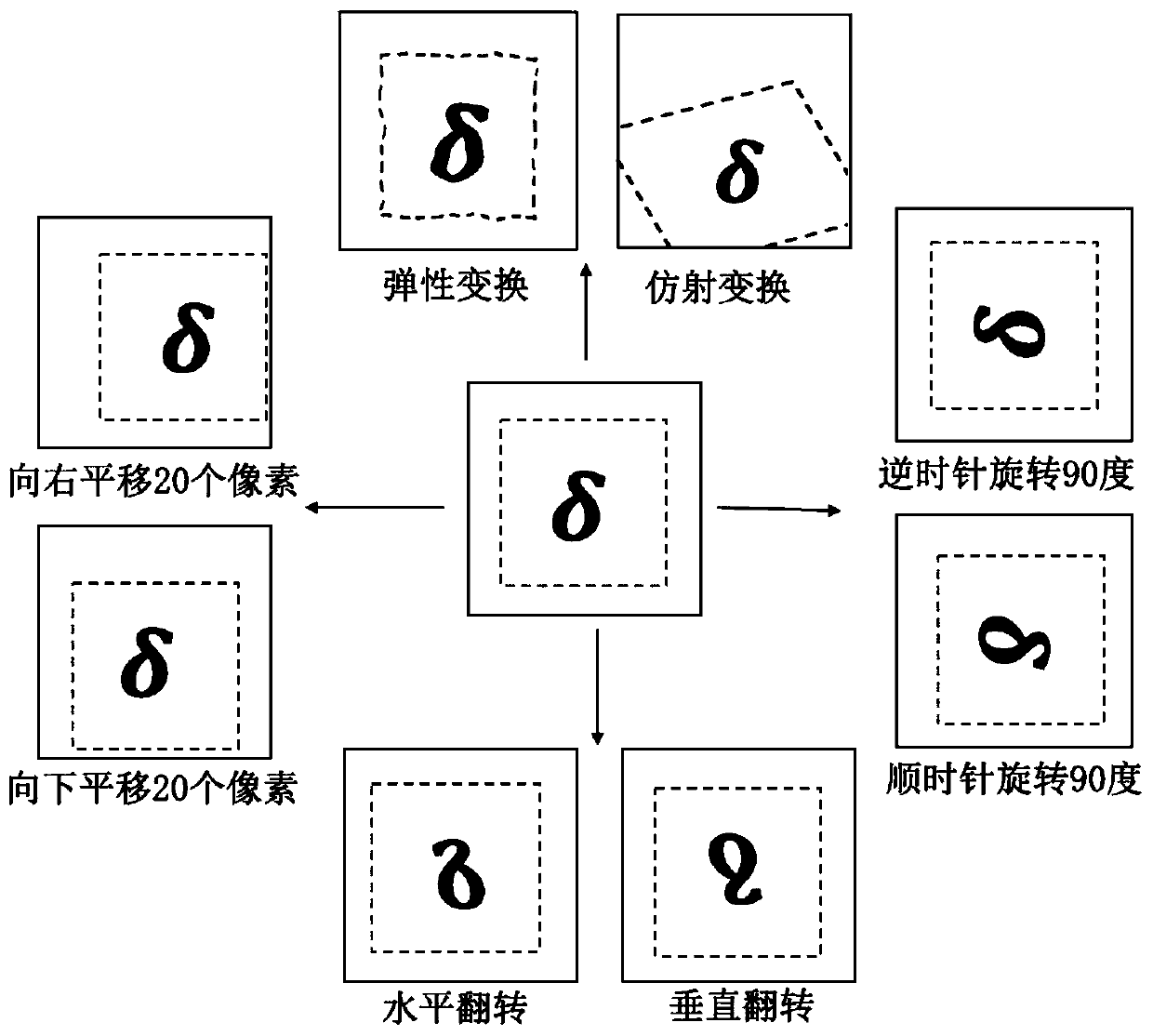
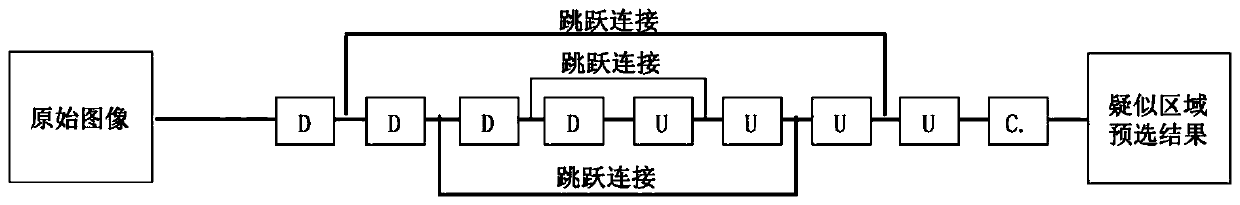
Intracranial aneurysm area automatic detection system and method for CTA image
PendingCN111445478AIncrease diversityReduce overfittingImage enhancementImage analysisNuclear medicineStereo image
The invention discloses an intracranial aneurysm area automatic detection system and a detection method for a CTA image. The system comprises: a data enhancement module which is used for carrying outenhancement treatment on collected CTA image data; a suspected area pre-selection module used for carrying out model training on the enhanced CTA image data and determining central coordinates of a suspected area; a fine target detection module used for cutting an original image according to the center coordinates, then carrying out model training, and marking the area where the intracranial aneurysm is located on the original image; a multi-dimensional fusion module used for cutting a three-dimensional image according to the center coordinates, then carrying out model training, marking the area where the intracranial aneurysm is located on the three-dimensional image, and marking the area where the intracranial aneurysm is located on the original image in combination with a fine target detection result; and an incremental training module used for acquiring a newly added CTA image and re-determining the area where the intracranial aneurysm is located.
Owner:JILIN UNIV
Micrograft for the treatment of intracranial aneurysms and method for use
A device for occluding a vasculature of a patient including a micrograft having an absorbent polymeric structure with a lumen of transporting blood. The micrograft has a series of peaks and valleys formed by crimping. The occluding device is sufficiently small and flexible to be tracked on a guidewire and / or pushed through a microcatheter to a site within the vasculature of the patient. Delivery systems for delivering the micrografts are also disclosed.
Owner:NEUROGAMI MEDICAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com