A feeder cell-free culture medium and system
A technology of feeding cells and culture medium, applied in the field of cell culture, can solve the problem of not understanding the function of MEF
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] Example 1: Analysis of the in vitro microenvironment of human embryonic stem cells
[0192] Materials and Method
[0193] Mouse embryo fibroblast cell culture
[0194] MEF (SCRC-1046 cell line, Cryosite, Lane Cove, Sydney, NSW, AUS) at 80cm 2 Amplify 6 generations in culture flask (Nalge Nunc International, Rochester, NY, USA), use 85% Dulbecco's Modification of Eagle's medium (DMEM) (Invitrogen, MountWaverley, VIC, Australia), add 10% fetus Bovine Serum (FBS) (Invitrogen), 2x10 -3 M L-glutamine (Invitrogen) and 1000IU / mL penicillin / streptomycin (Invitrogen), 5% CO 2 , 37°C. Subsequently, mitomycin-C (Sigma-Aldrich, CastleHill, NSW, AUS) was added to the flask containing MEF, and the cells were incubated at 37°C, 5% CO 2 Incubate for 2.5 to 3 hours. Give petri dish (10cm 2 ) (Nalge Nunc International) cover 0.1% gelatin (Sigma-Aldrich), add MEF at least 1 hour later. MEF cells at 20,000 cells / cm 2 Inoculate 10cm covered with 0.1% gelatin (Sigma-Aldrich) 2 (Nalge Nunc Intern...
Embodiment 2
[0241] Example 2: Proteomics analysis of keratinocyte conditioned medium cultured in vitro
[0242] The purpose of this study is to comprehensively examine the in vitro microenvironment of keratinocytes. Specifically, proteomics methods were used to identify the main factors required for the growth of keratinocytes produced by feeder cells. In addition, using the above-mentioned serum-free medium, the medium is fully defined and has a very low protein content. The significant advantage of the extremely low protein content of the serum-free medium is that it does not "mask" the main factors secreted by feeder cells that can support the growth of keratinocytes. In addition, serum-containing media usually require pre-processing steps before performing proteomic analysis, such as "Multiple Affinity Removal System" (MARS) (Agilent Technologies). The MARS immune depletion technology involves the removal of high-abundance proteins in serum-containing media, which may result in the los...
Embodiment 3
[0277] Example 3: Growth of hES cells without feeder cells and serum
[0278] The hES cells were grown and tested in the following medium formulation: 1ug / mL IGF-I / 1-64VN chimeric protein, 0.1ug / mL bFGF, 35ng / mL activin-A and 40μg / mL laminin.
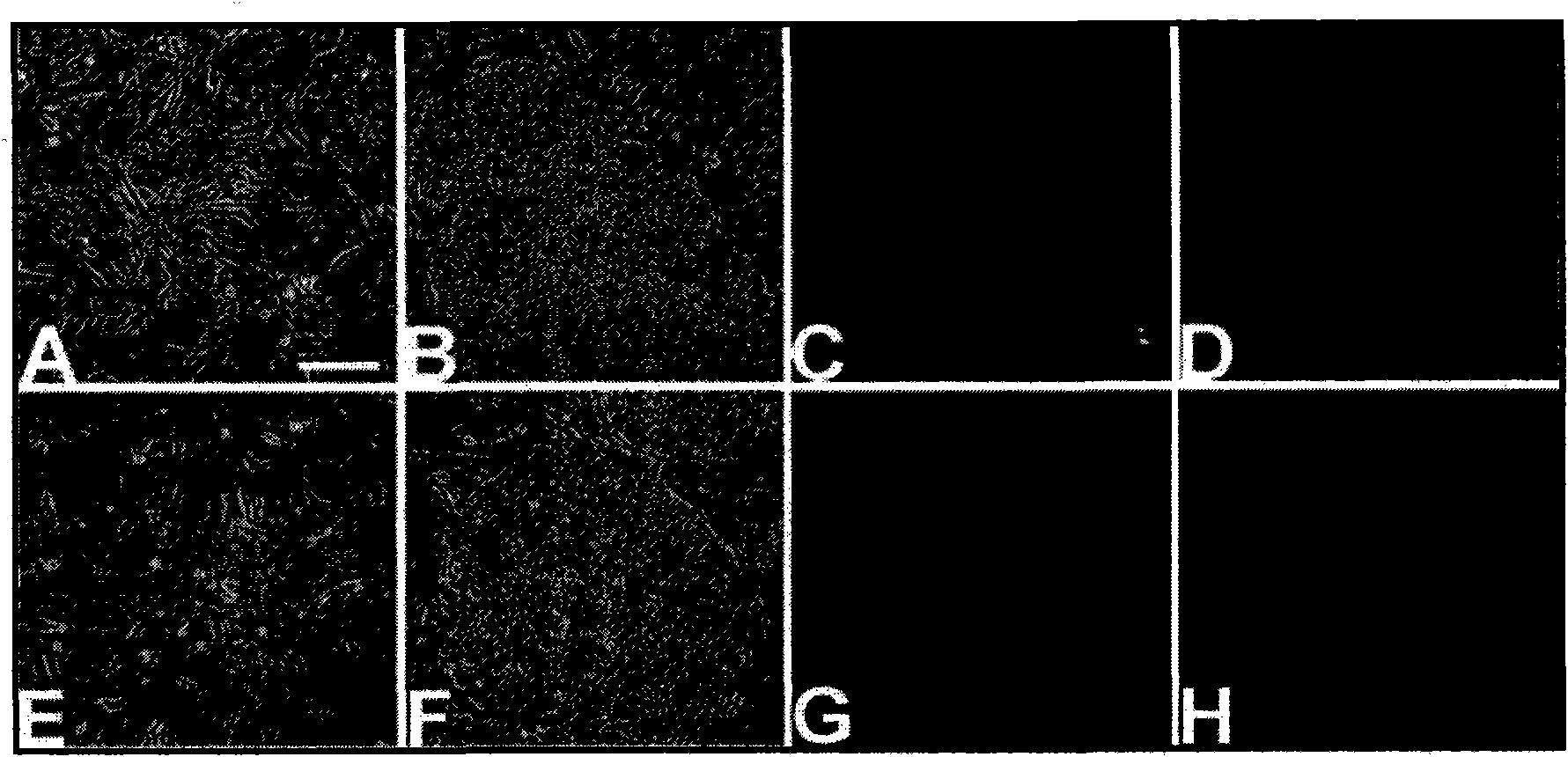
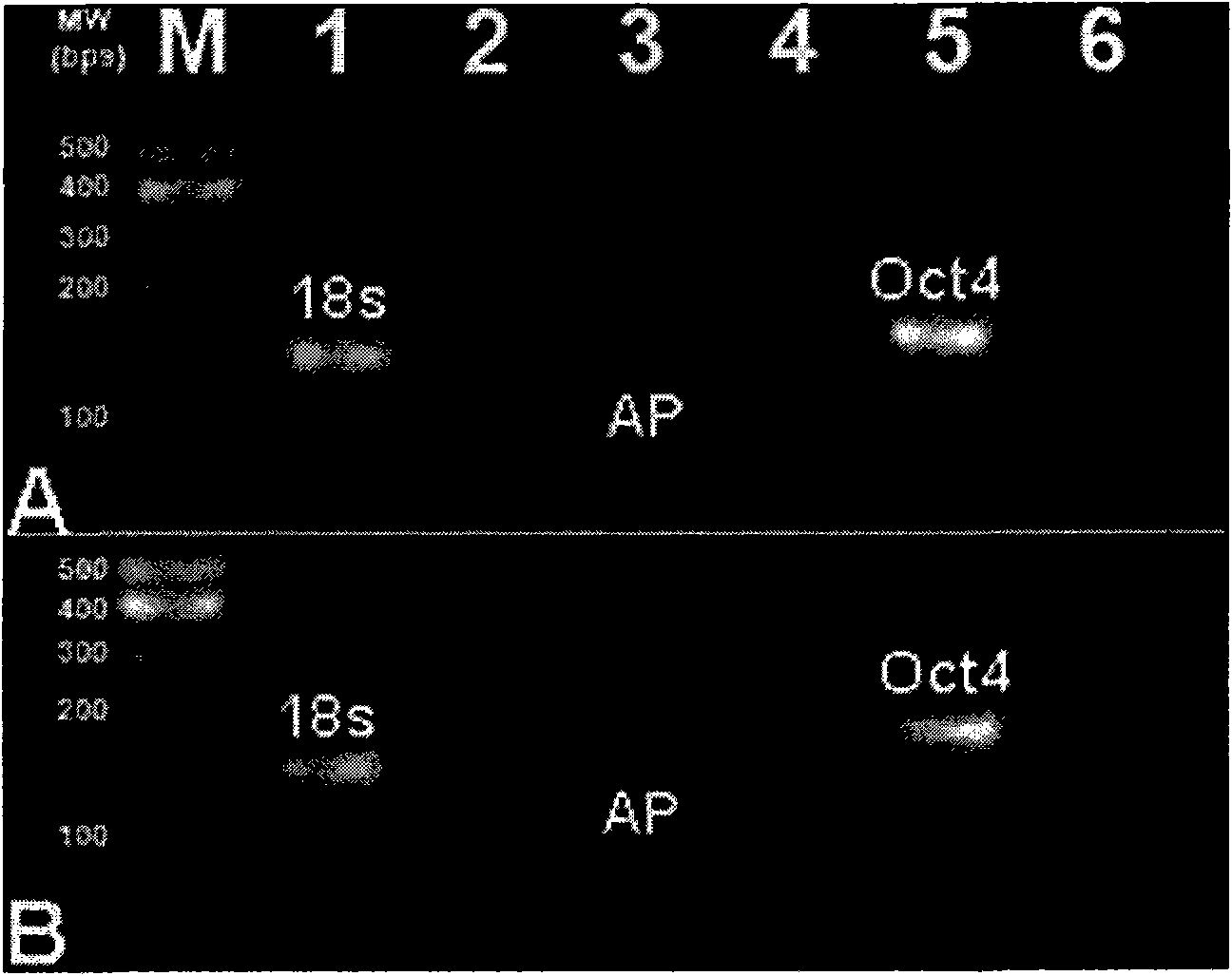
[0279] Immunofluorescence (IF) was performed using antibodies against Oct4, TRA1-60, SSEA-4, SSEA-1 antigens. IF Research ( Figure 8 ) Confirmed the expression of Oct4, TRA1-60 and SSEA-4, but only low expression of SSEA-1. hES cells also showed a large nuclear-to-cytoplasmic ratio, representing the hES phenotype.
[0280] Rex1 is abnormal, and this result confirms that it is significantly down-regulated in our culture system. However, when examining Oct4 and Nanog, these amplicons in our culture system showed an almost 2-fold increase in expression ( Picture 9 ).
[0281] Together, these data suggest that the system can indeed maintain these cells in an "undifferentiated state."
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com