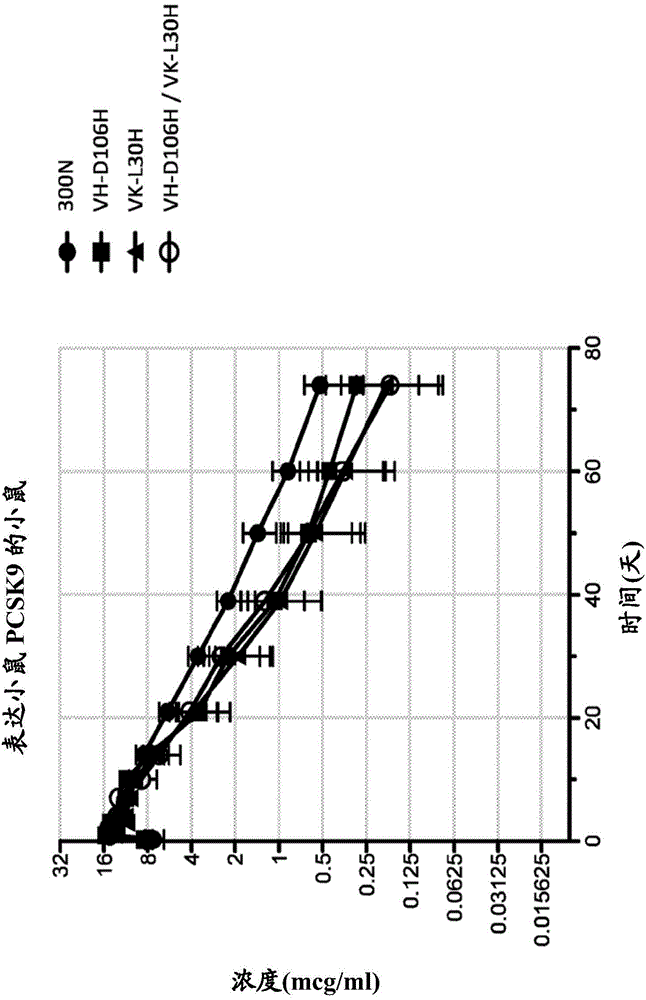
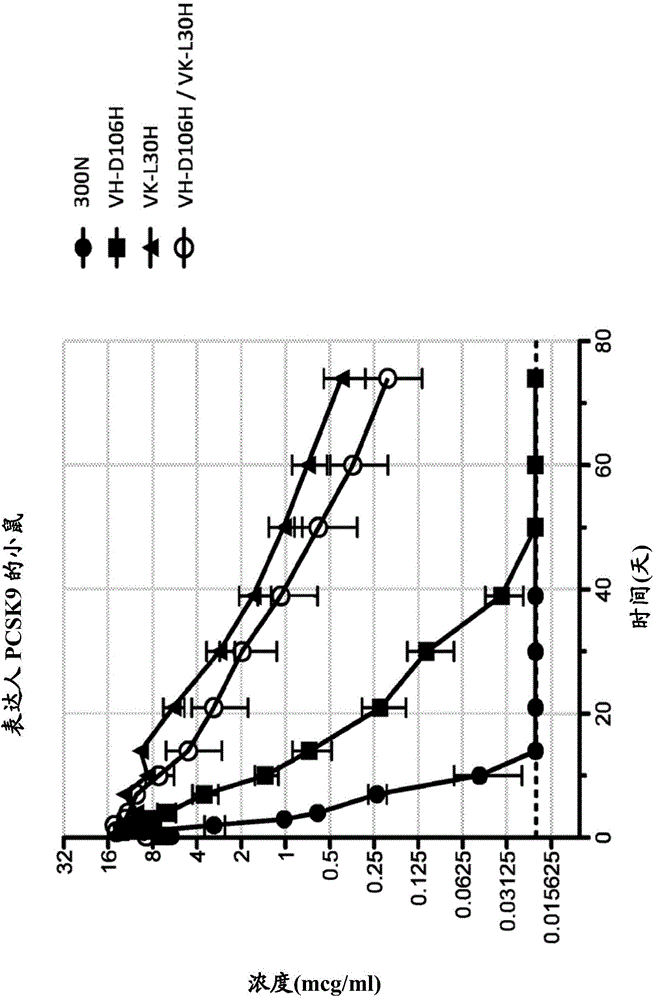
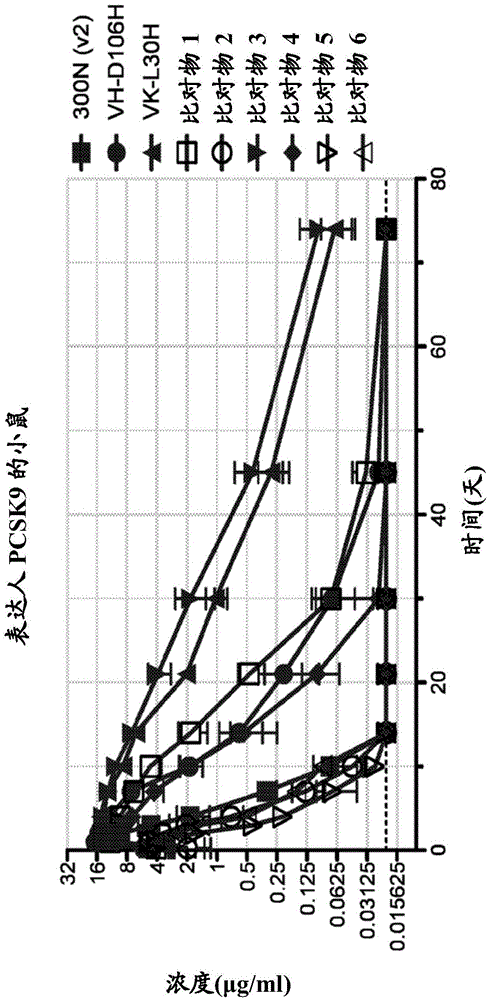
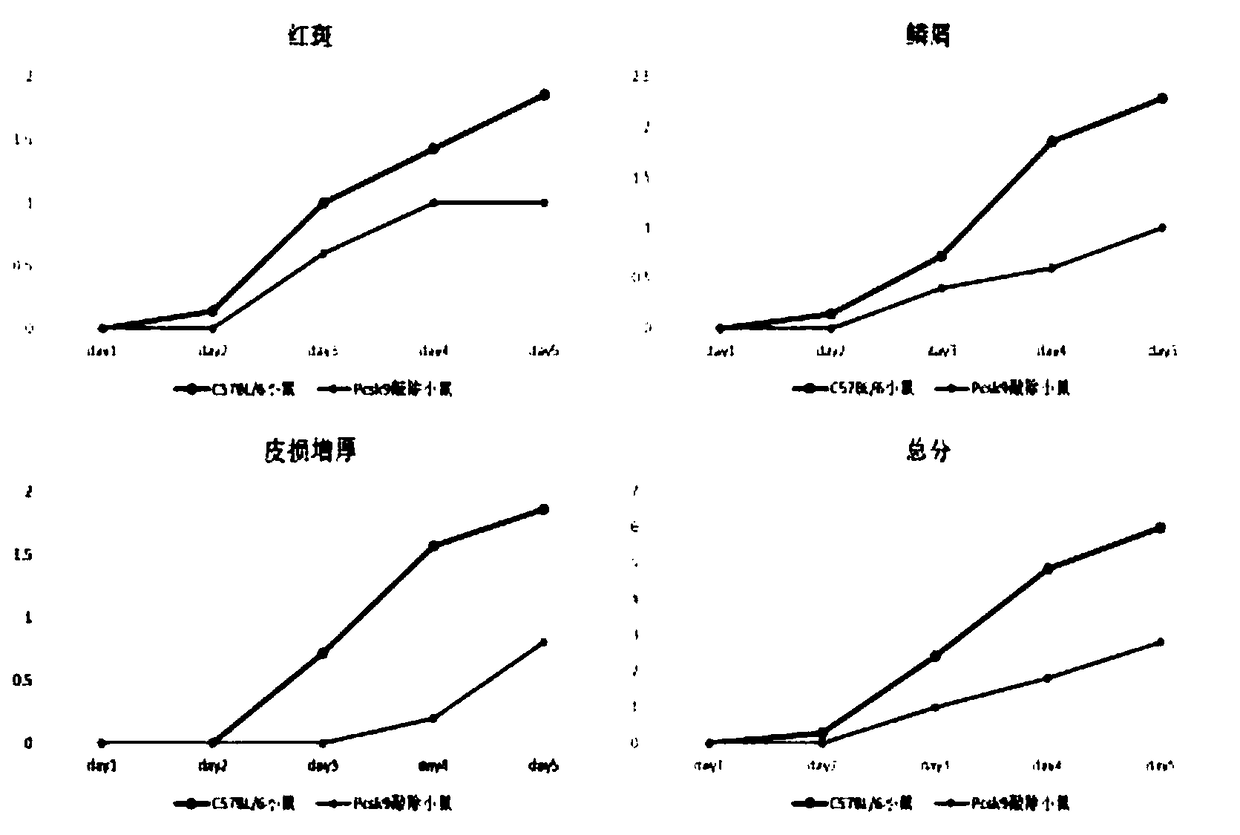
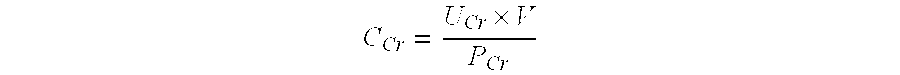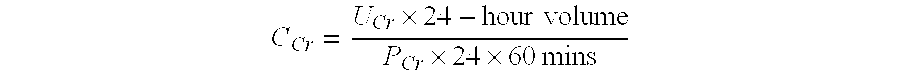Patents
Literature
106 results about "Prohormone Convertases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
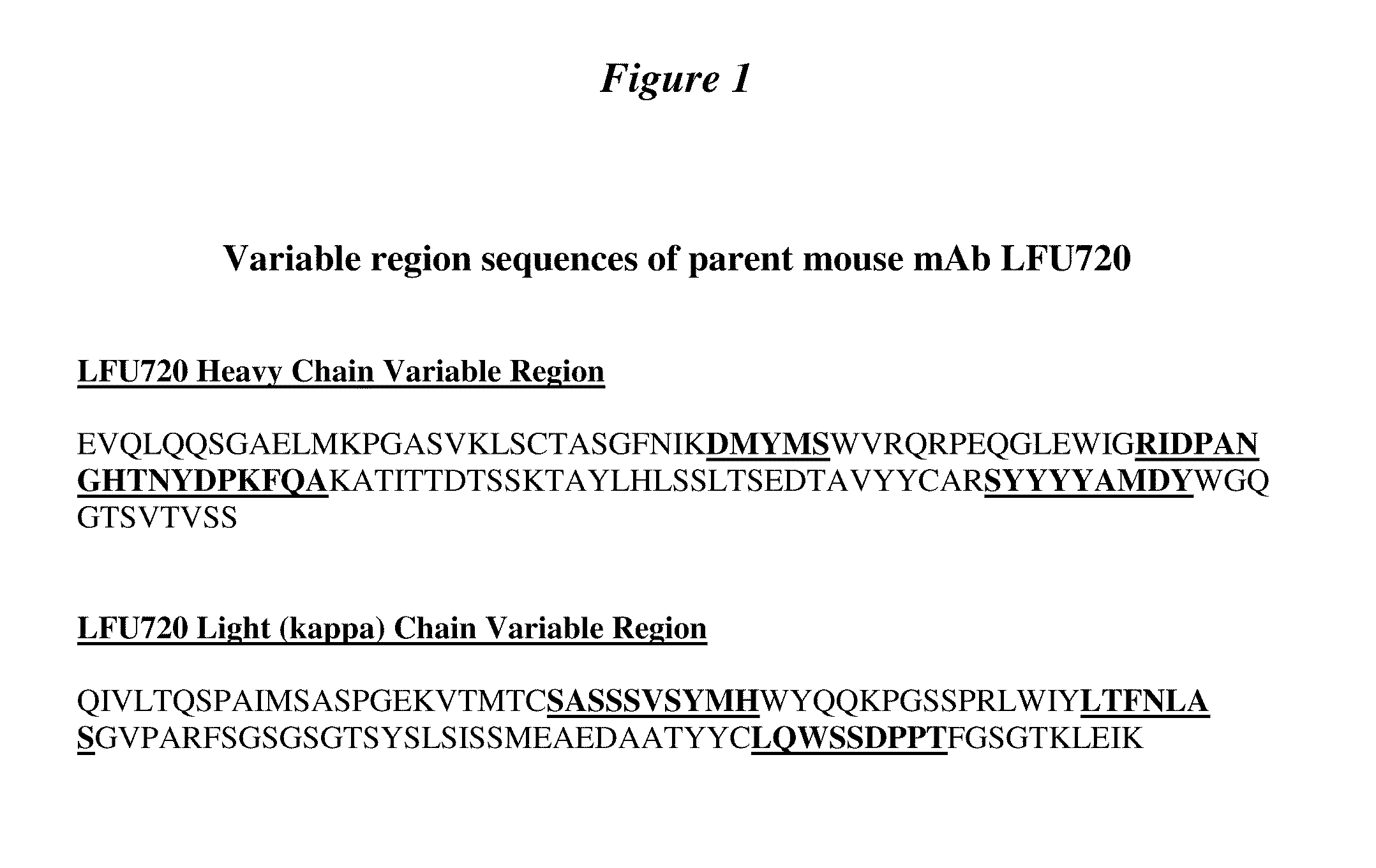
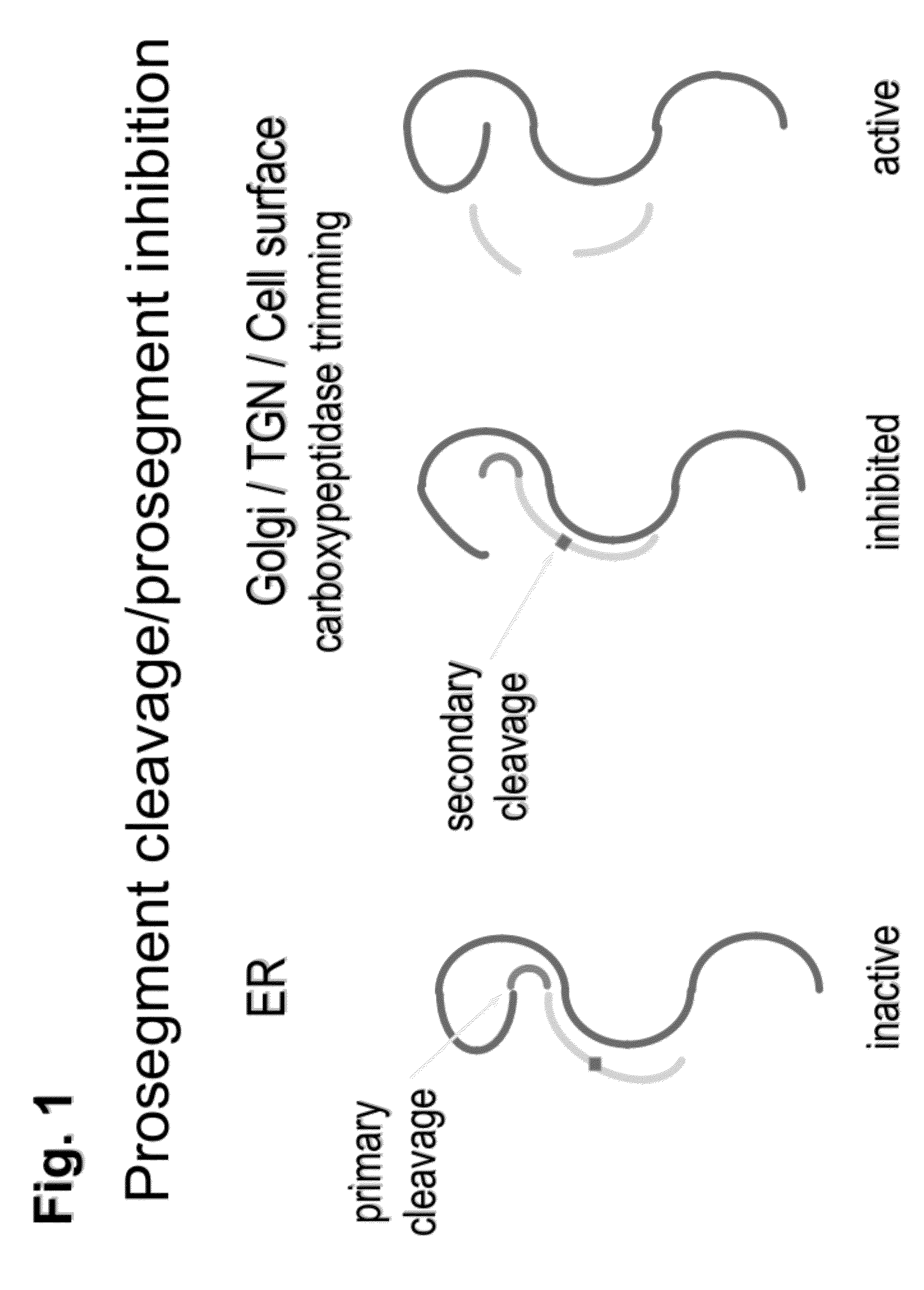
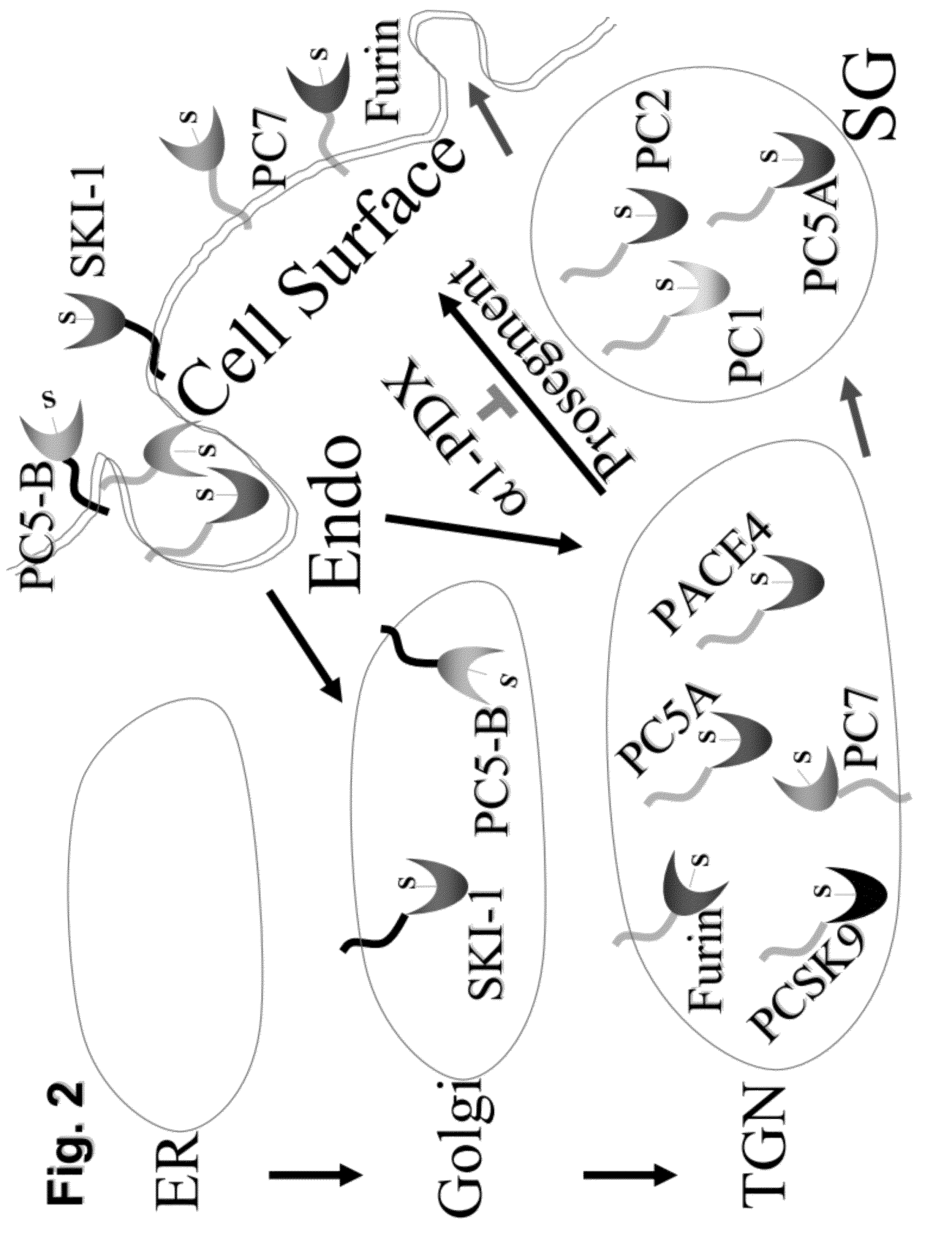
Prohormone Convertases. The two proprotein convertases that specialize in the processing of the precursors of peptide hormones and neuropeptides are also known in the field as "prohormone convertases". Both "prohormone convertase" and "proprotein convertase" are interchangeably abbreviated as "PC".
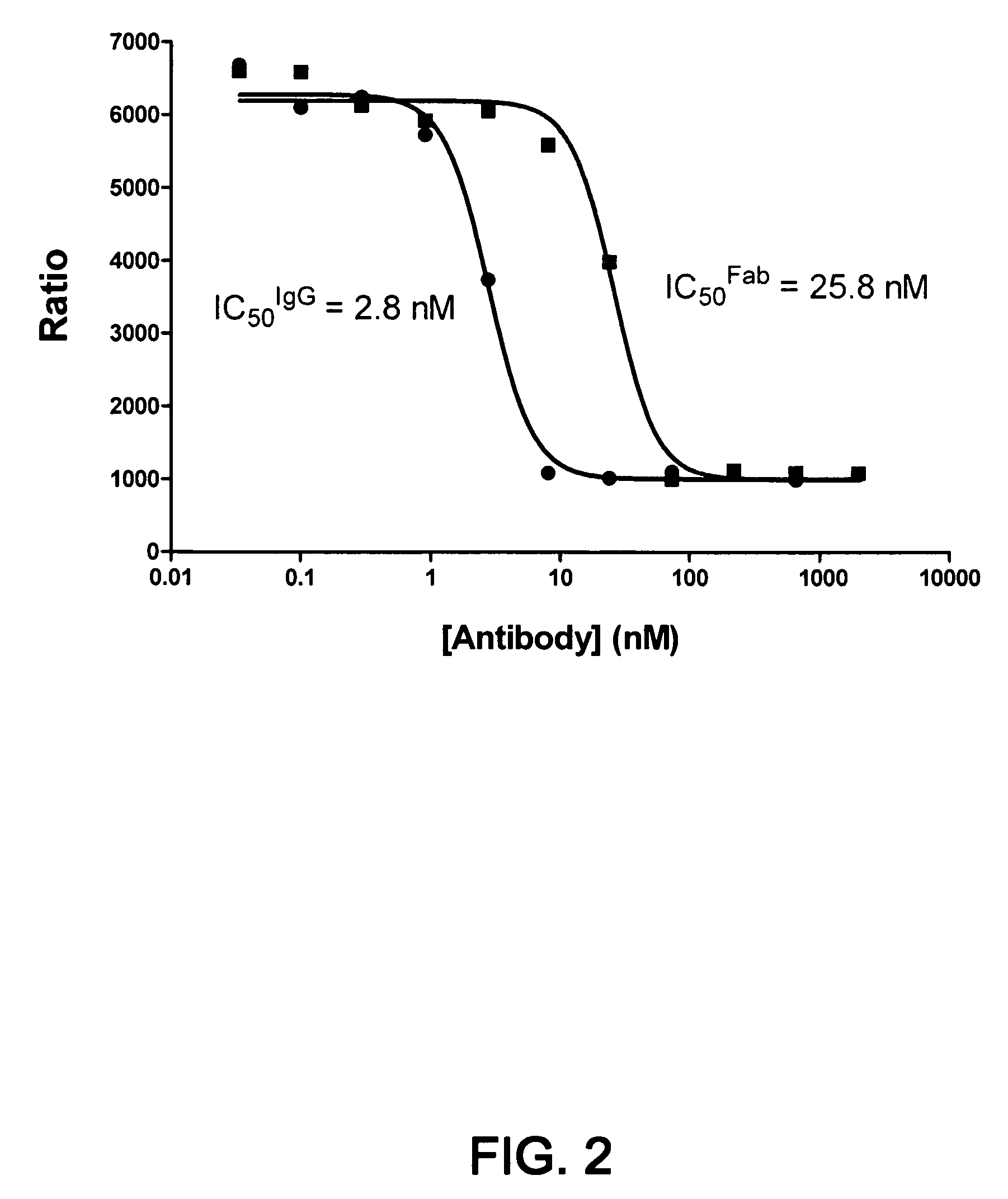
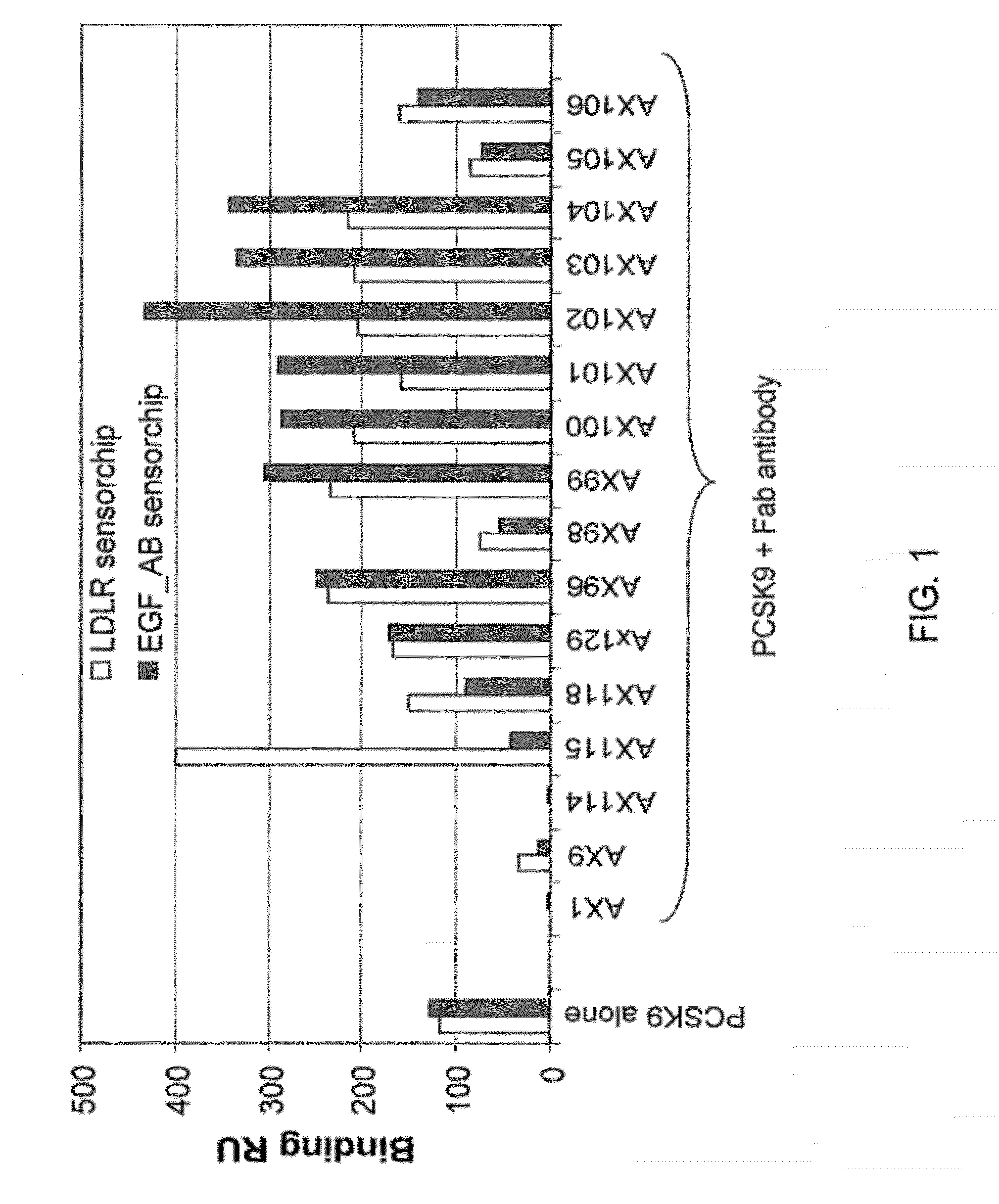
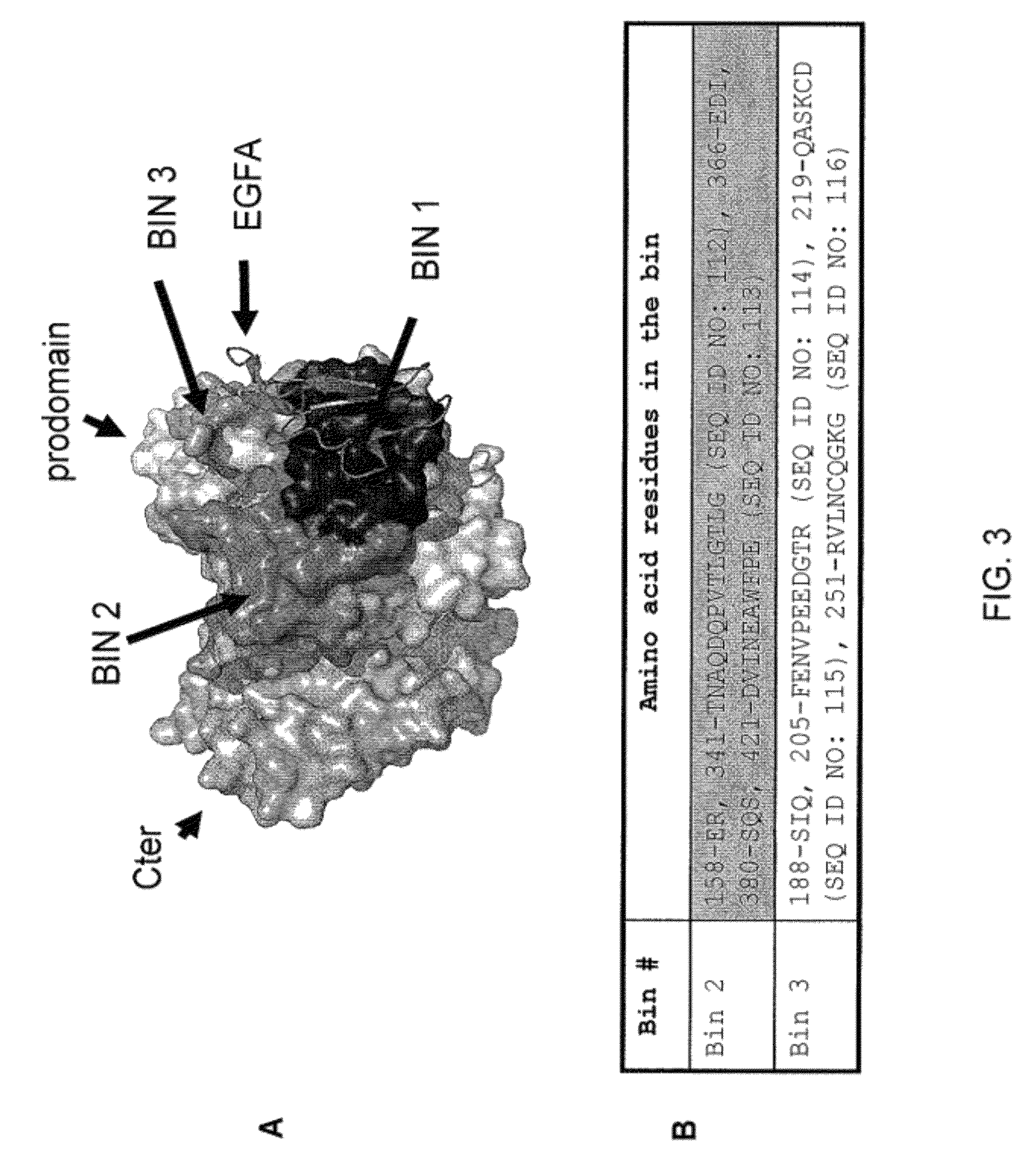
Isolated antibody which specifically binds to PCSK9
Owner:PFIZER INC RINAT NEUROSCIENCE CORP
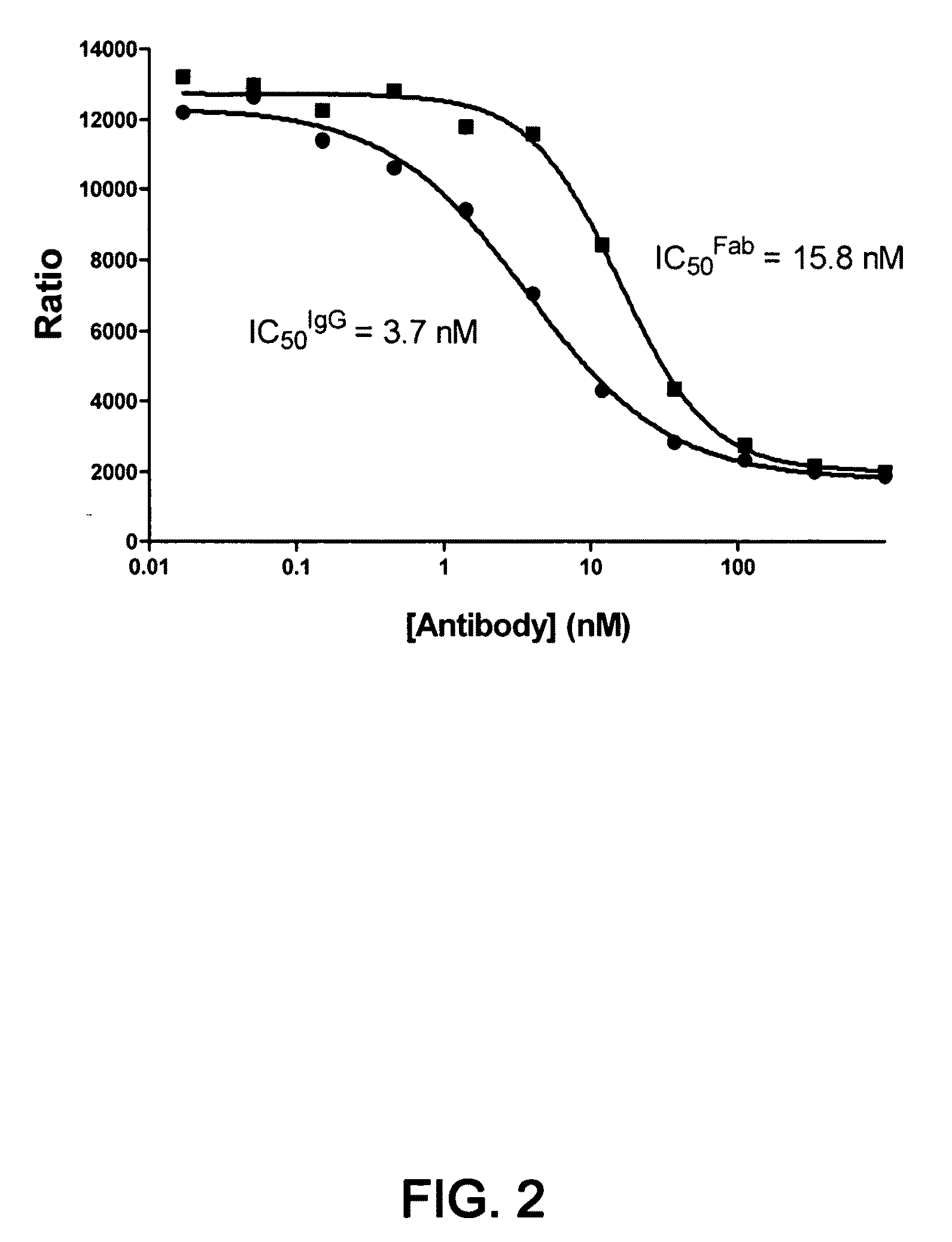
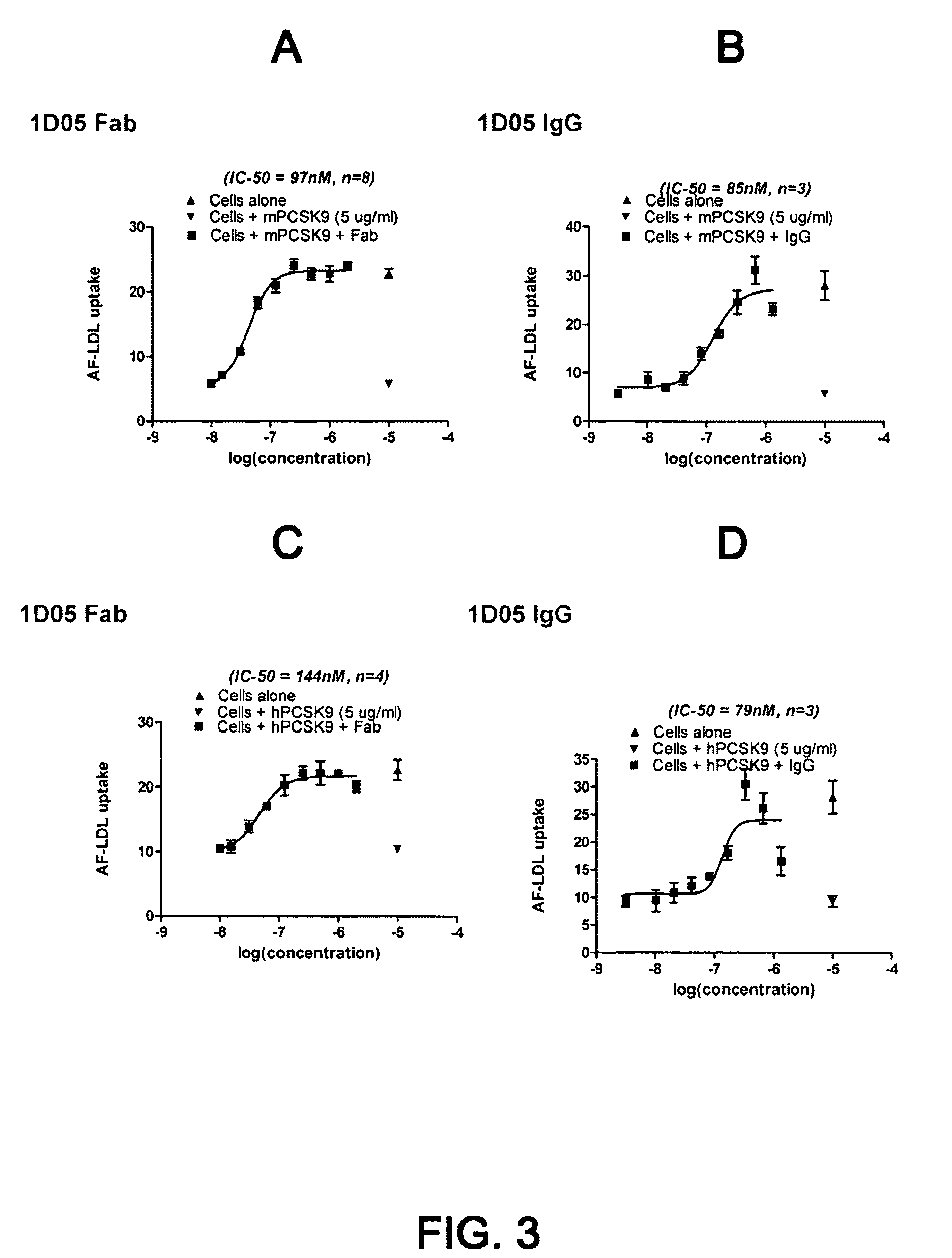
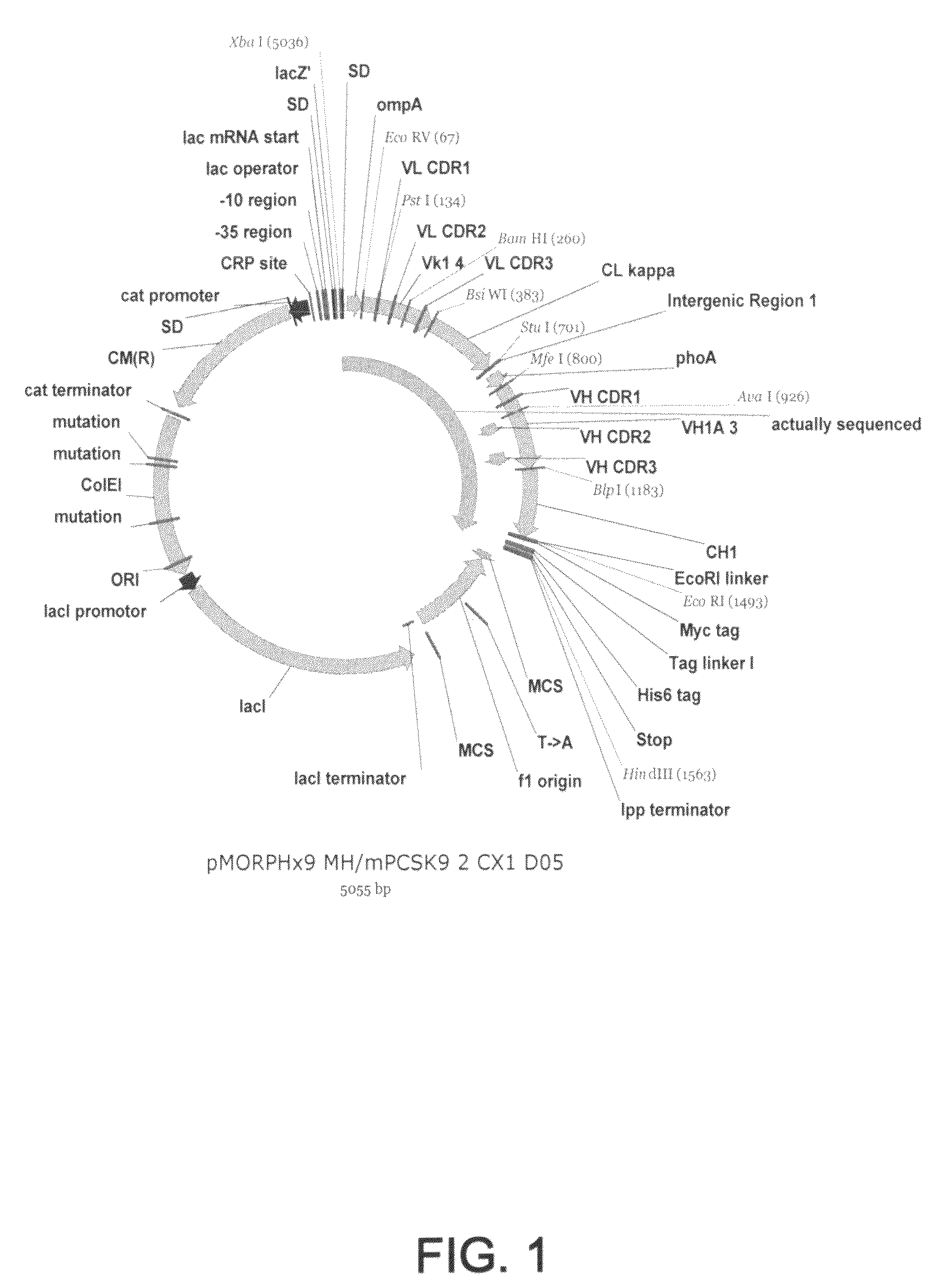
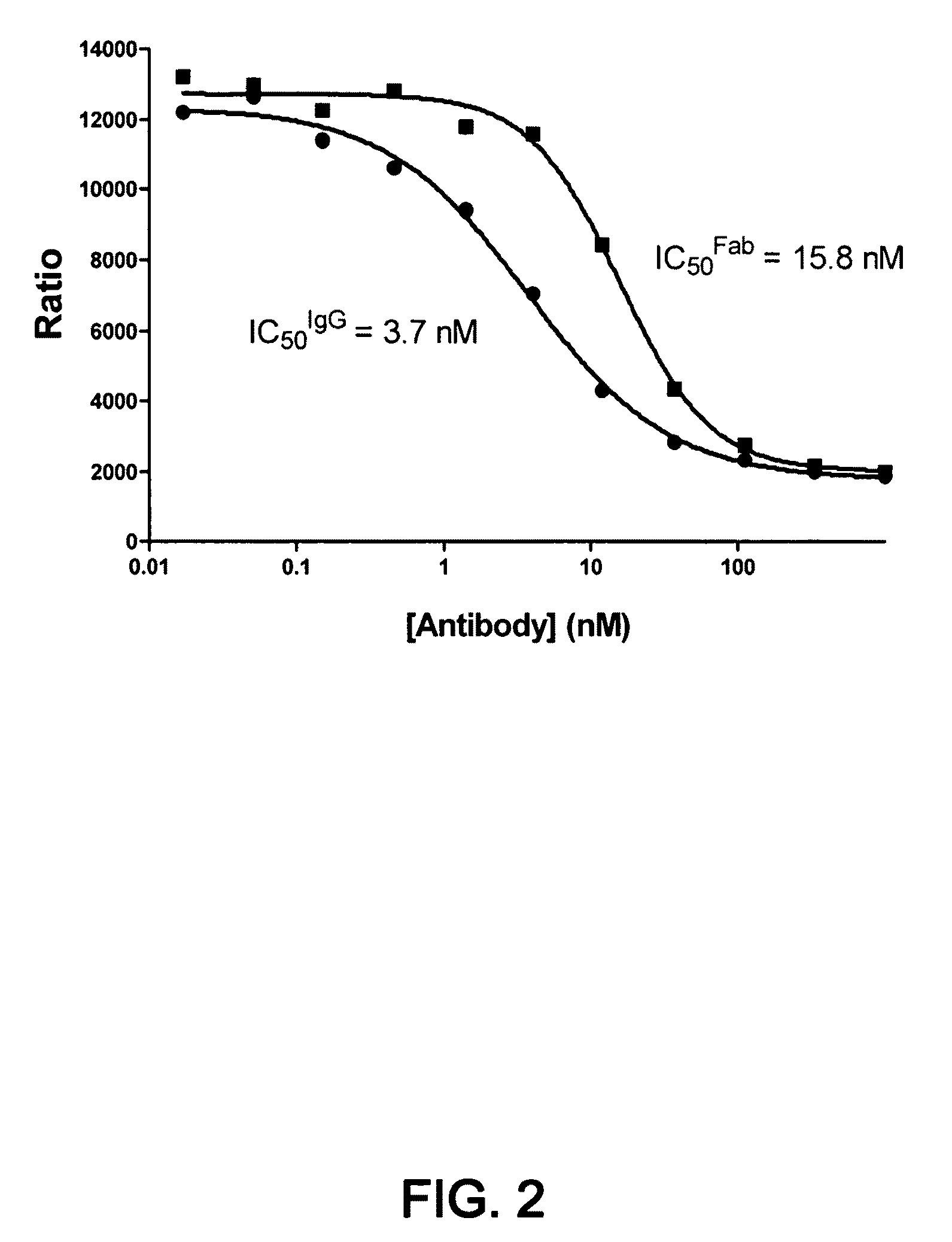
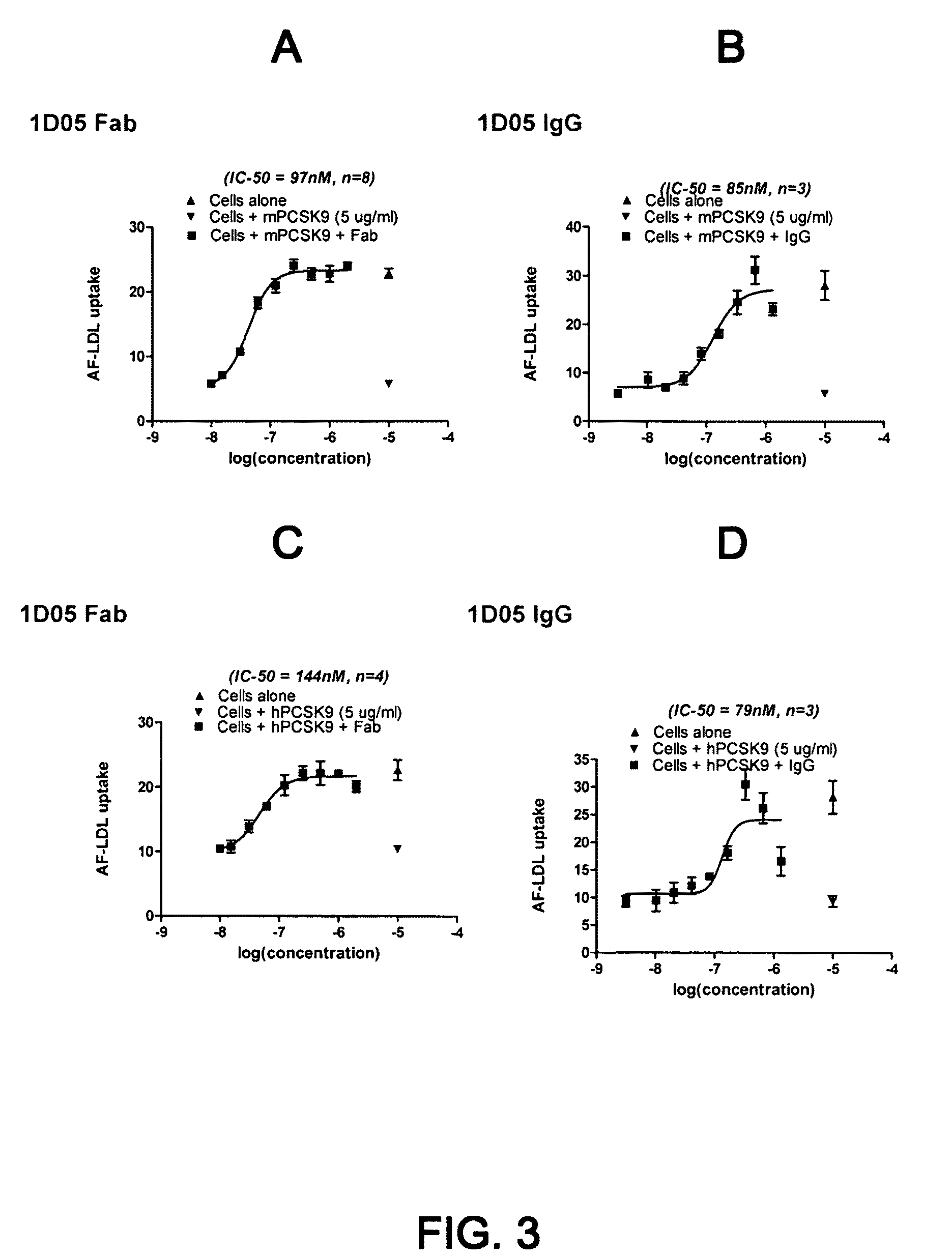
1D05 PCSK9 antagonists
ActiveUS20090246192A1Lower Level RequirementsFermentationVector-based foreign material introductionKexinSubtilisin
Antagonists of human proprotein convertase subtilisin-kexin type 9 (“PCSK9”) are disclosed. The disclosed antagonists are effective in the inhibition of PCSK9 function and, accordingly, present desirable antagonists for use in the treatment of conditions associated with PCSK9 activity. The present invention also discloses nucleic acid encoding said antagonists, vectors, host cells, and compositions comprising the antagonists. Methods of making PCSK9-specific antagonists as well as methods of using the antagonists for inhibiting or antagonizing PCSK9 function are also disclosed and form important additional aspects of the present disclosure.
Owner:MERCK SHARP & DOHME LLC +1
1D05 PCSK9 antagonists
Antagonists of human proprotein convertase subtilisin-kexin type 9 (“PCSK9”) are disclosed. The disclosed antagonists are effective in the inhibition of PCSK9 function and, accordingly, present desirable antagonists for use in the treatment of conditions associated with PCSK9 activity. The present invention also discloses nucleic acid encoding said antagonists, vectors, host cells, and compositions comprising the antagonists. Methods of making PCSK9-specific antagonists as well as methods of using the antagonists for inhibiting or antagonizing PCSK9 function are also disclosed and form important additional aspects of the present disclosure.
Owner:MERCK SHARP & DOHME LLC +1
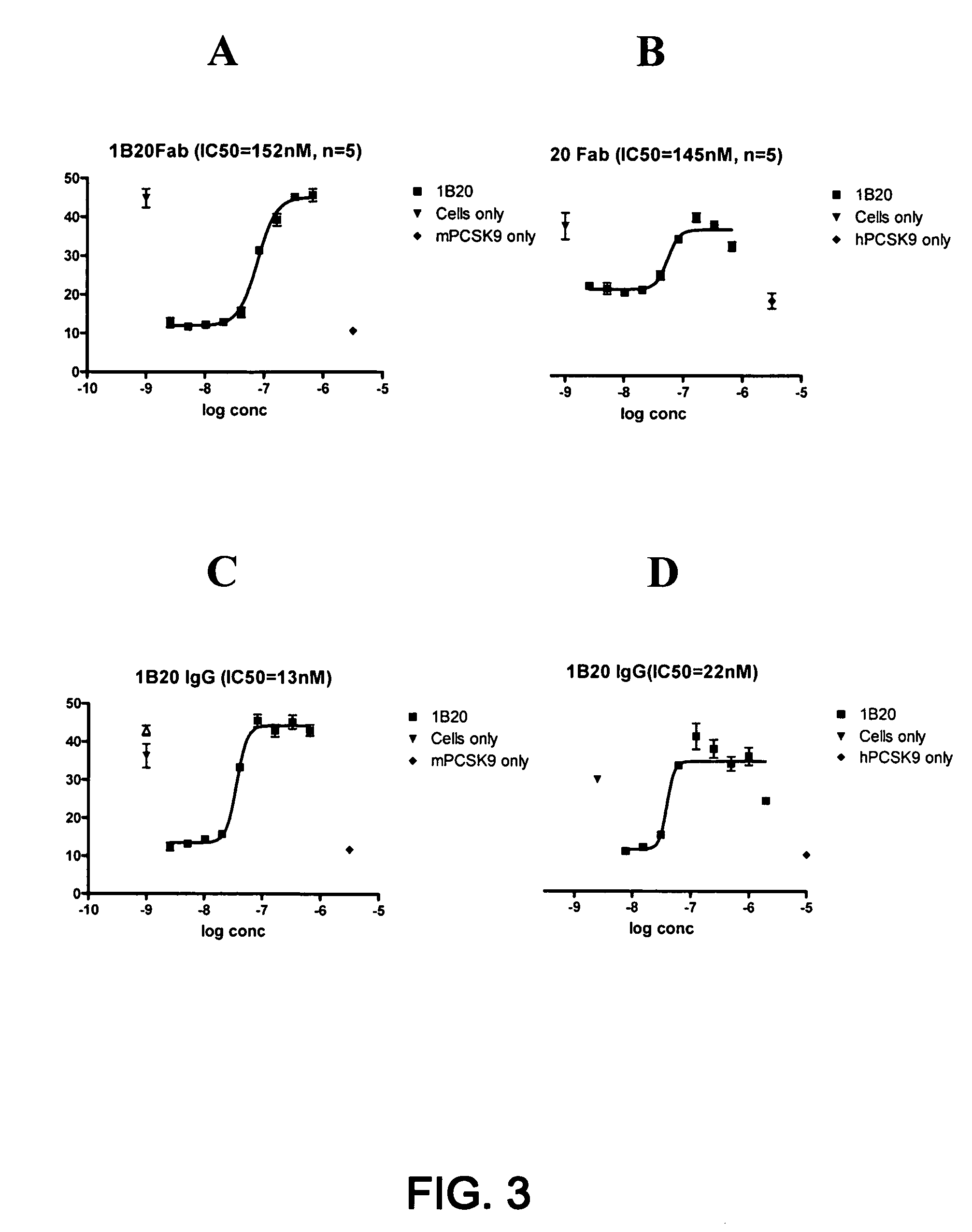
1B20 PCSK9 antagonists
Antagonists of human proprotein convertase subtilisin-kexin type 9 (“PCSK9”) are disclosed. The disclosed antagonists are effective in the inhibition of PCSK9 function and, accordingly, present desirable antagonists for use in the treatment of conditions associated with PCSK9 activity. The present invention also discloses nucleic acid encoding said antagonists, vectors, host cells, and compositions comprising the antagonists. Methods of making PCSK9-specific antagonists as well as methods of using the antagonists for inhibiting or antagonizing PCSK9 function are also disclosed and form important additional aspects of the present disclosure.
Owner:MERCK SHARP & DOHME LLC
Pcsk9 antagonists
ActiveUS20110142849A1Reduce severitySufficient amountPeptide/protein ingredientsGenetic material ingredientsKexinSubtilisins
The present invention provides antibody antagonists against proprotein convertase subtilisin / kexin type 9a (“PCSK9”) and methods of using such antibodies.
Owner:NOVARTIS AG
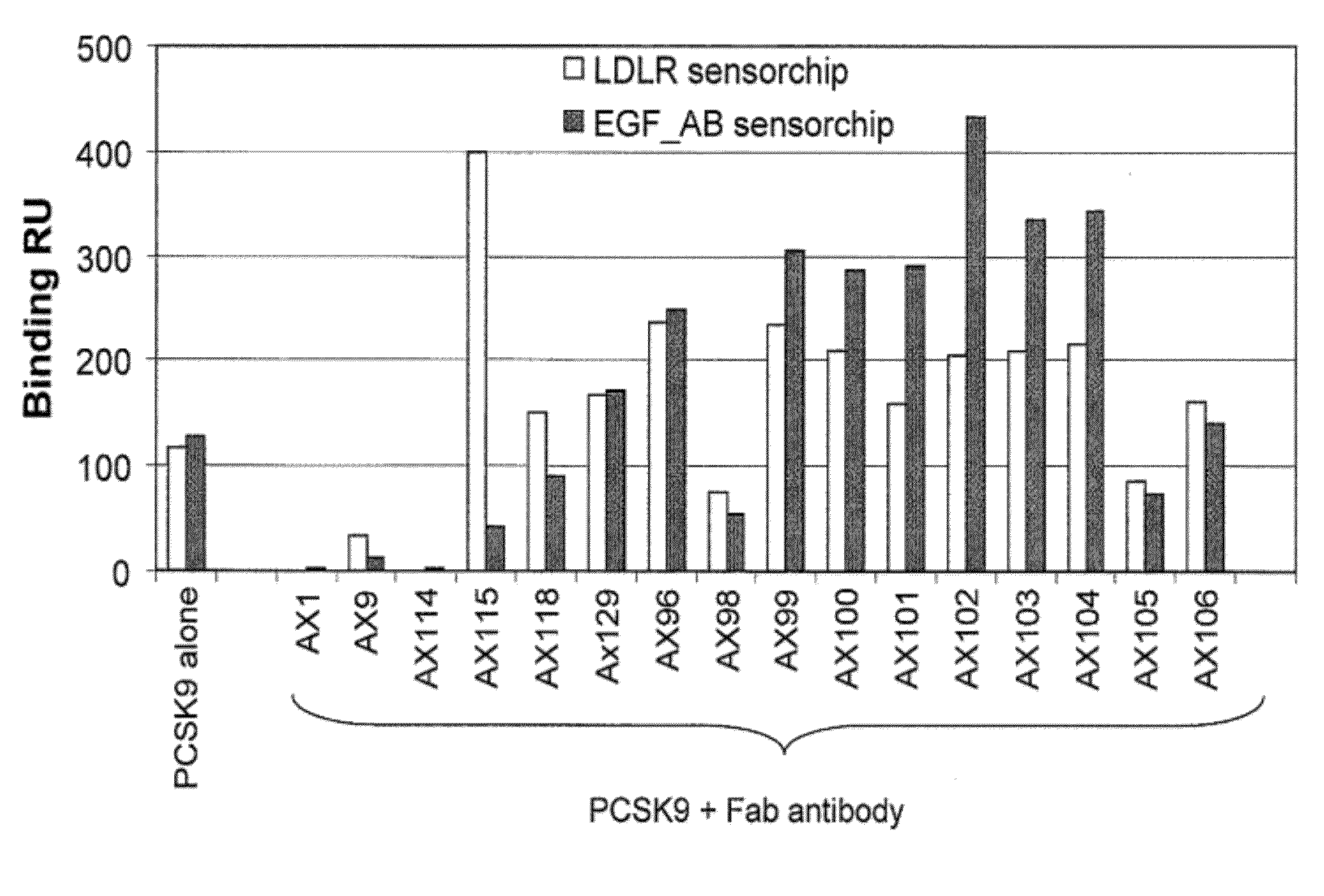
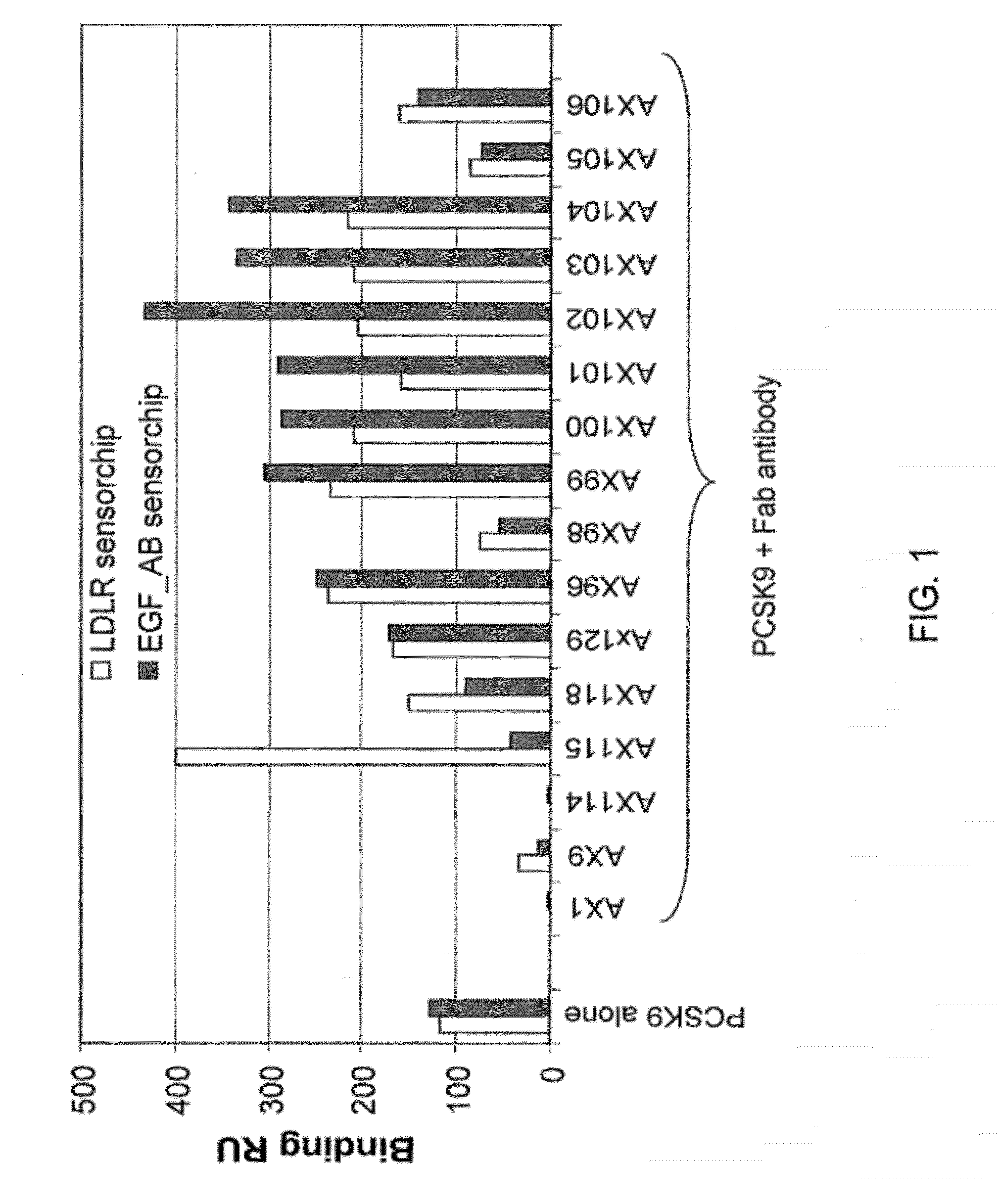
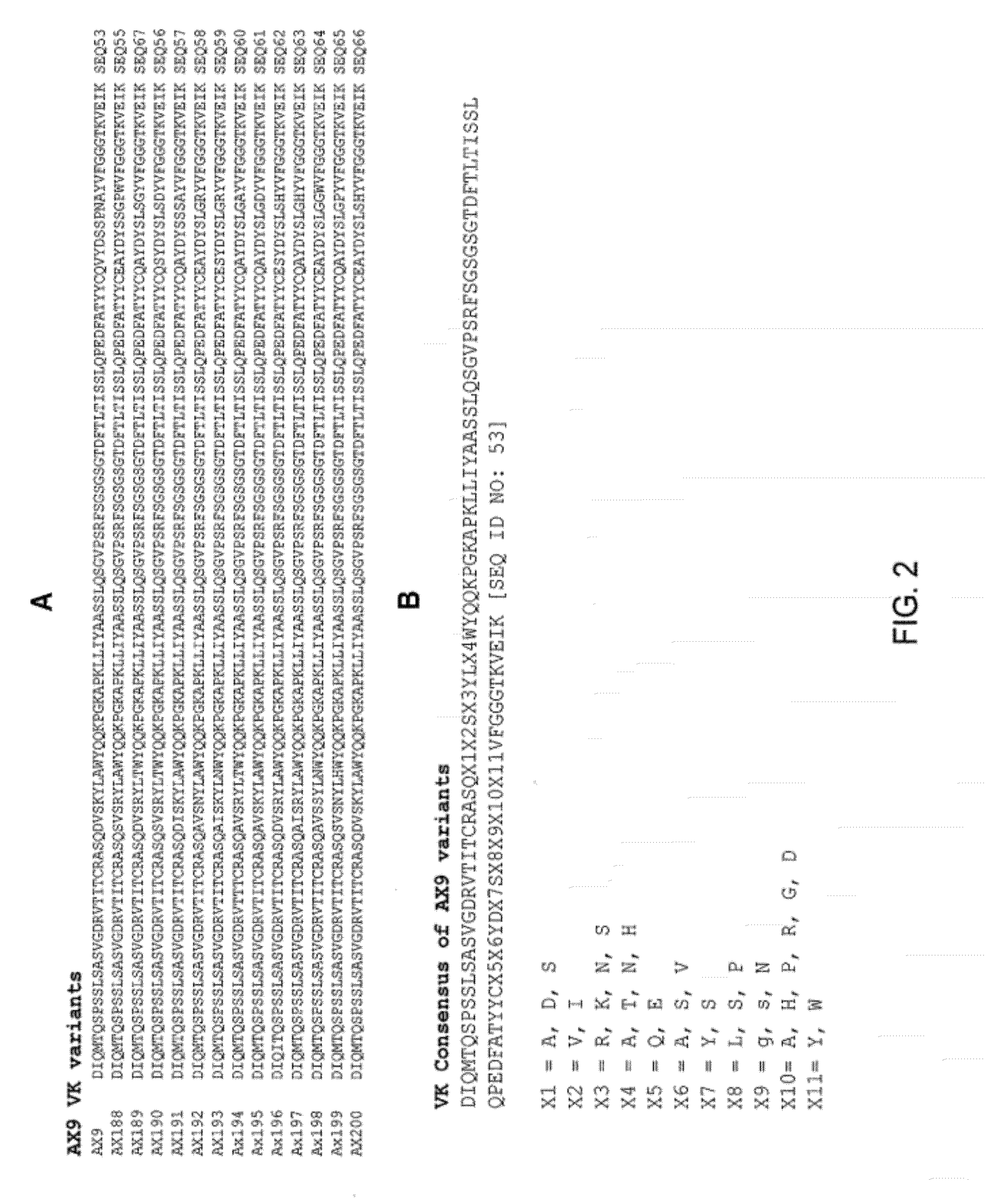
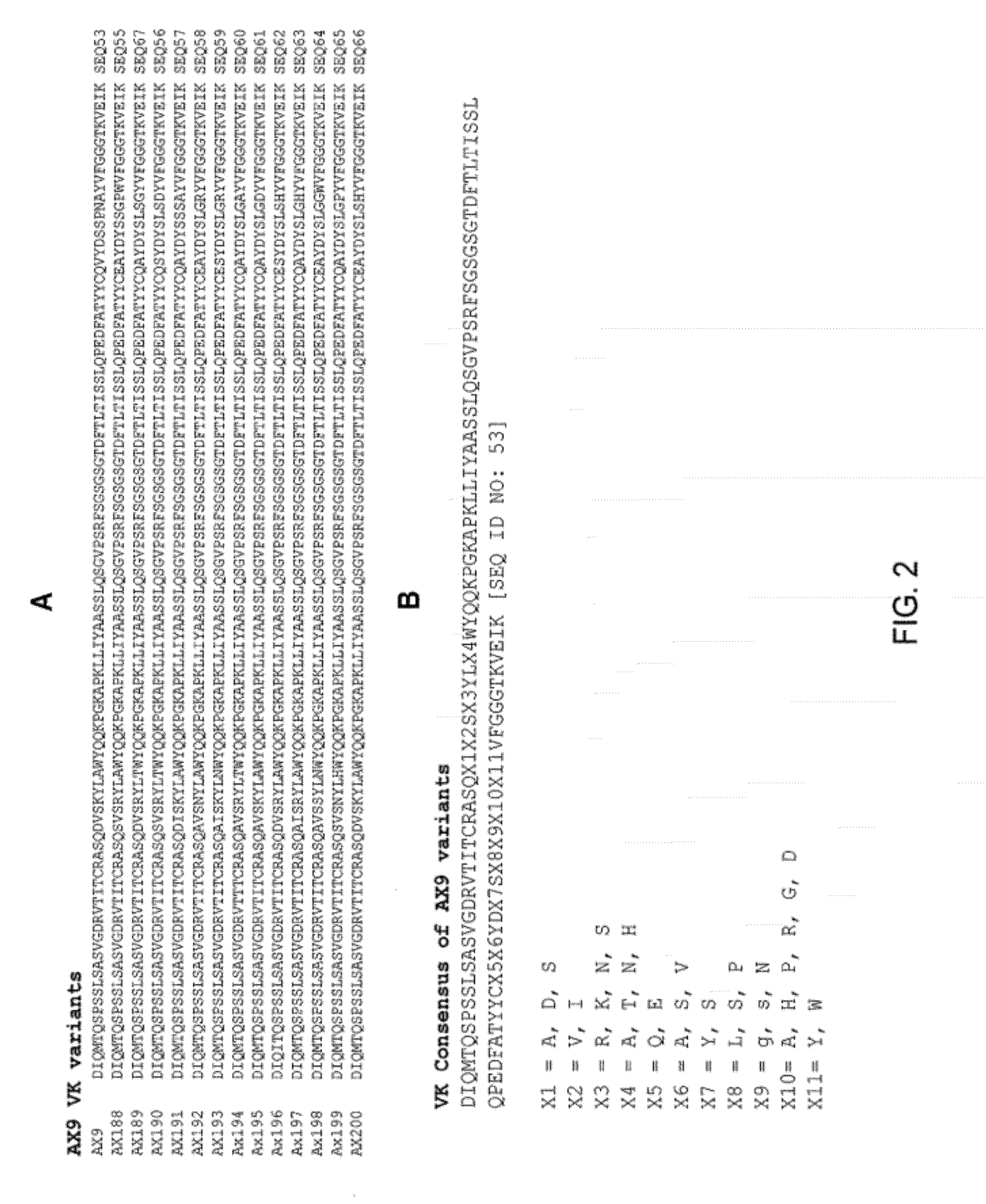
Ax1 and ax189 psck9 antagonists and variants
Antagonists of human proprotein convertase subtilisin-kexin type 9 (“PCSK9”) are disclosed. The disclosed antagonists are effective in the inhibition of PCSK9 function and, accordingly, present desirable antagonists for use in the treatment of conditions associated with PCSK9 activity. The present invention also discloses nucleic acid encoding said antagonists, vectors, host cells, and compositions comprising the antagonists. Methods of making PCSK9-specific antagonists as well as methods of using the antagonists for inhibiting or antagonizing PCSK9 function are also disclosed and form important additional aspects of the present disclosure.
Owner:MERCK SHARP & DOHME LLC
ANTI-PCSK9 ANTIBODIES WITH pH-DEPENDENT BINDING CHARACTERISTICS
ActiveUS20140044730A1High affinityReduced binding affinityMetabolism disorderAntibody ingredientsDiseaseKexin
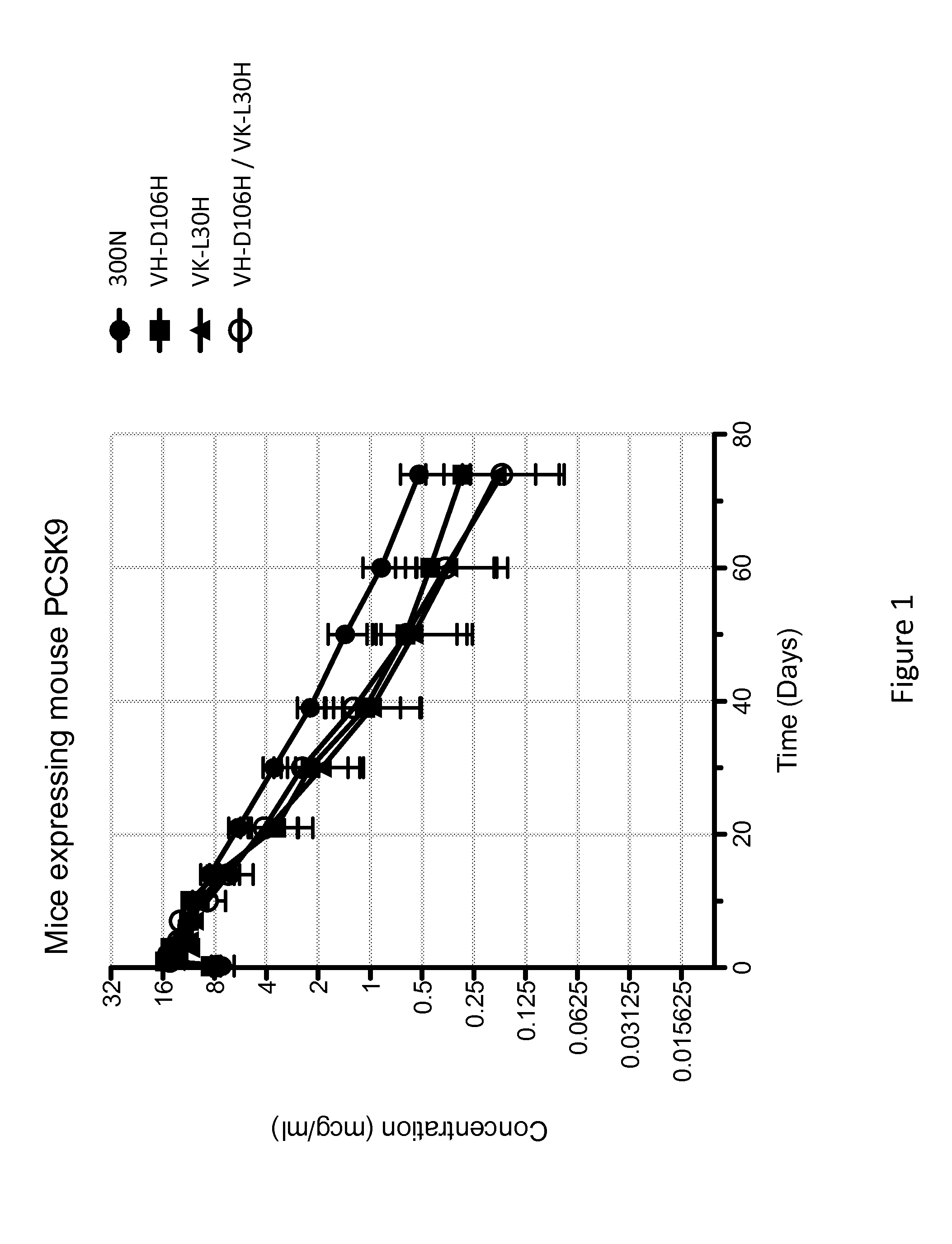
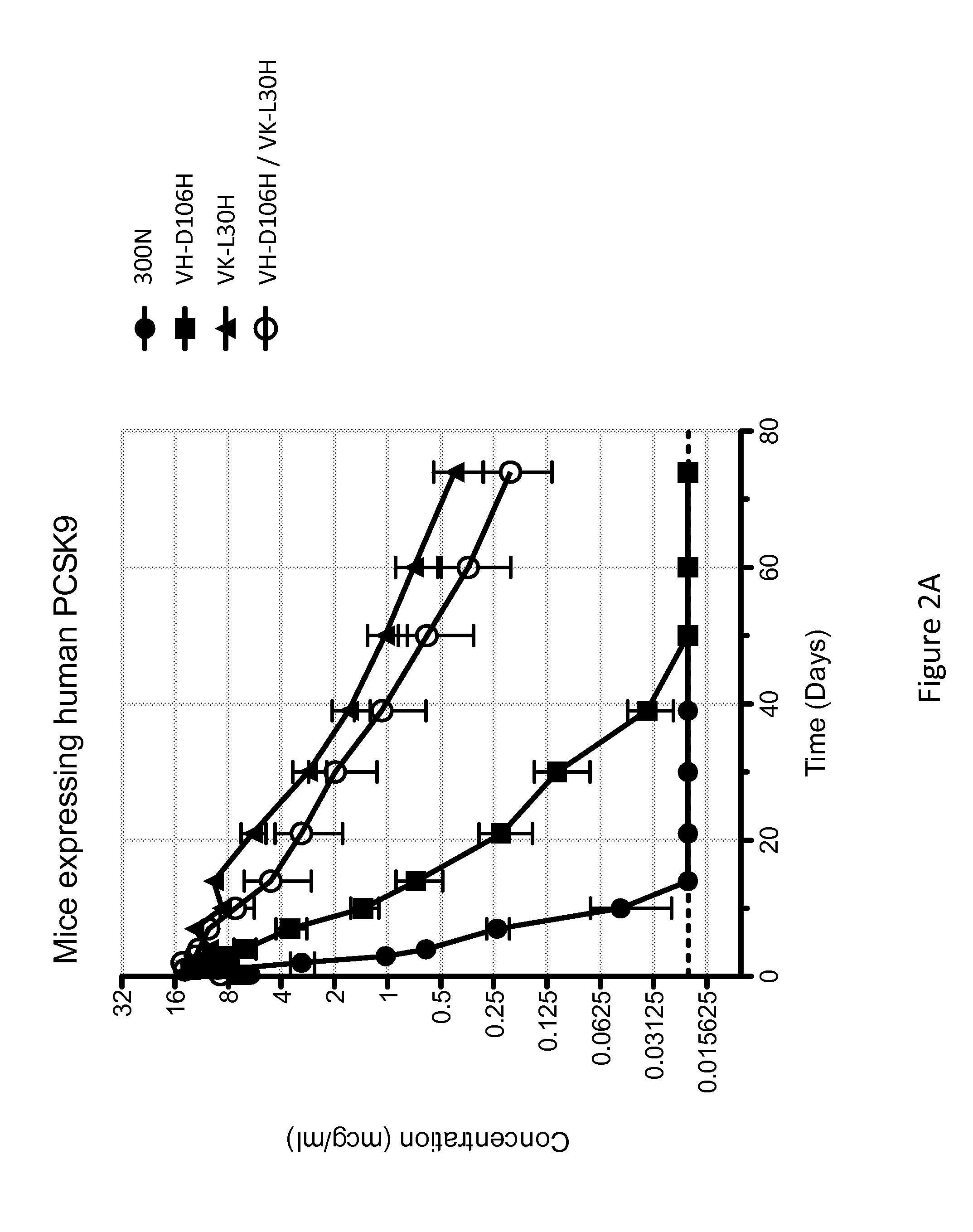
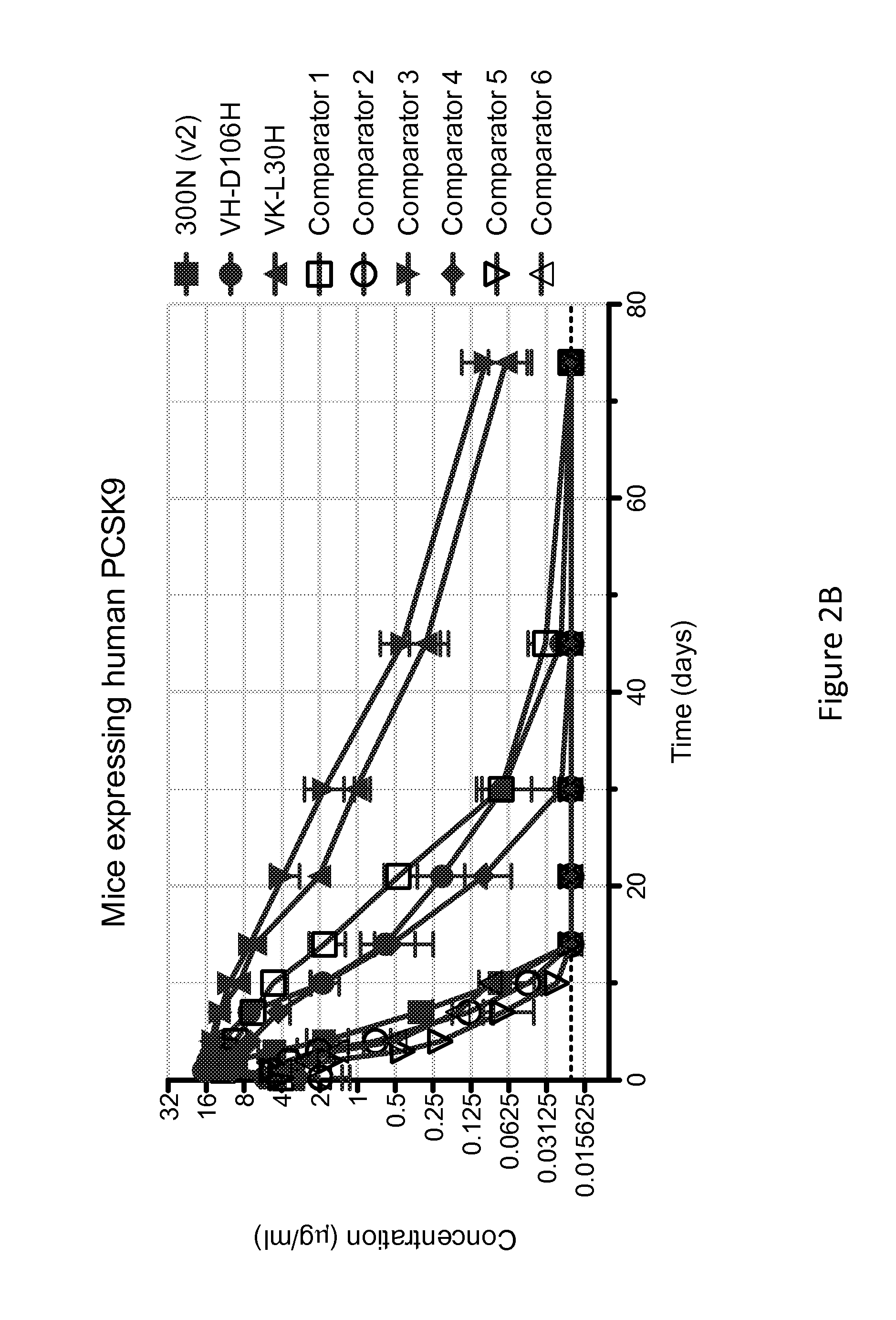
The present invention provides antibodies and antigen-binding fragments thereof that specifically bind proprotein convertase subtilisin / kexin-9 (PCSK9) with greater affinity at neutral pH than at acidic pH. The antibodies of the invention may possess one or more amino acid changes as compared to antibodies that do not exhibit pH-dependent binding properties. For example, the present invention includes anti-PCSK9 antibodies which possess one or more histidine substitutions in one or more complementarity determining regions. The antibodies of the invention, with pH-dependent binding properties, remain in circulation and exhibit cholesterol lowering activity for prolonged periods of time in animal subjects as compared to anti-PCSK9 antibodies that do not exhibit pH-dependent binding properties. The antibodies of the invention are therefore useful for treating diseases and disorders related to elevated HDL cholesterol, wherein the antibodies of the invention can be administered to a patient at a lower dose and / or with less frequent dosing as compared to antibodies that do not exhibit pH-dependent binding properties.
Owner:REGENERON PHARM INC
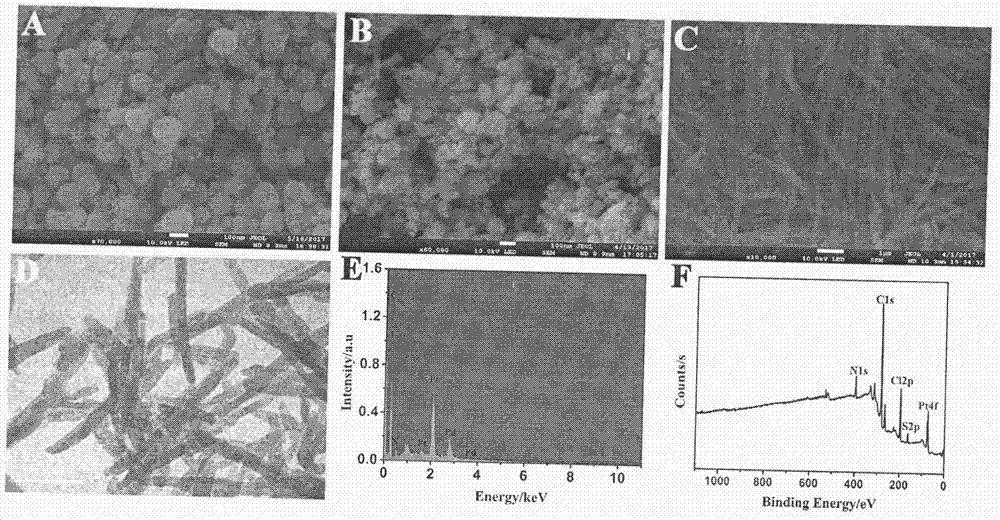
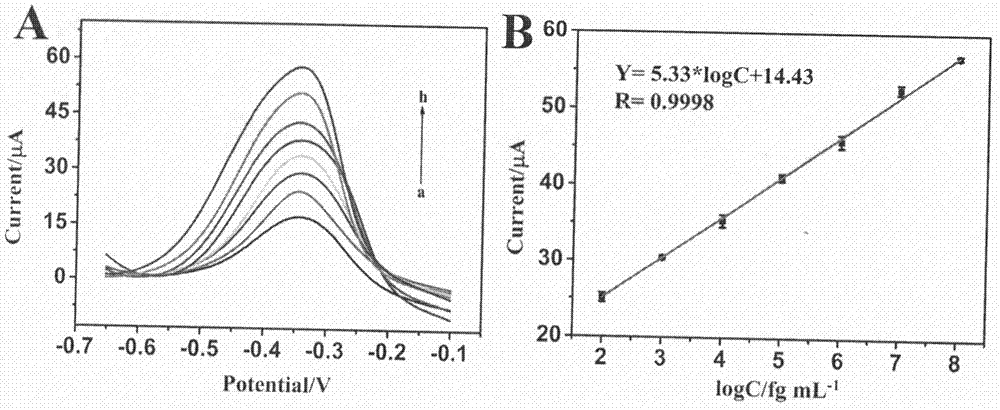
Method for preparing electrochemical immunosensor for pcsk9 protein detection
InactiveCN107389949AImprove conductivityHigh sensitivityBiological testingMaterial electrochemical variablesProtein detectionStaphylococcus aureus
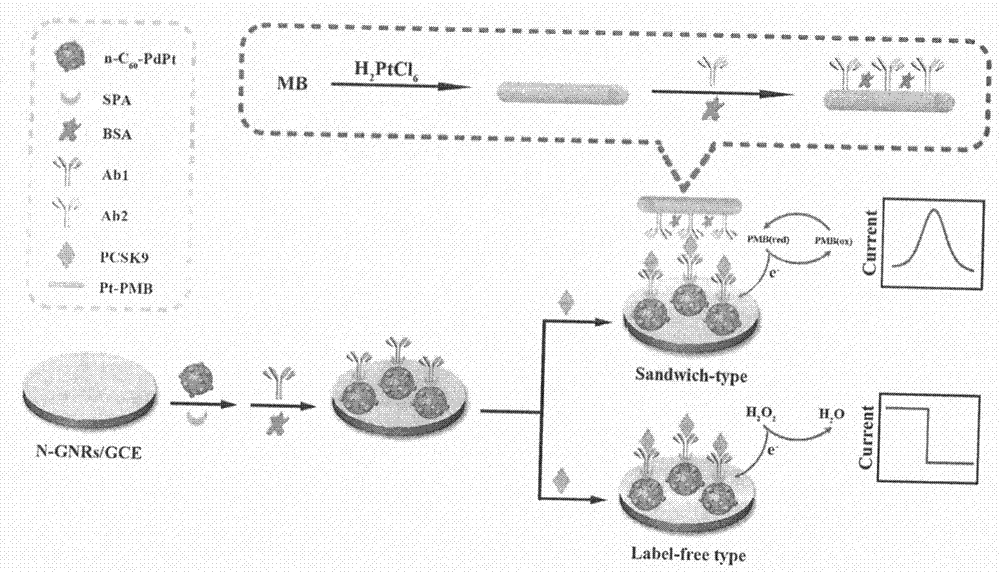
The invention relates to a preparation method and application of an electrochemical immunosensor for a biomarker, namely a recombinant proprotein convertase subtilisin kexin type 9 (pcsk9) protein for predicting and diagnosing cardiovascular diseases, and belongs to the technical field of electrochemical detection. The preparation method is characterized by comprising the following steps: firstly, performing nitrogen doping on a graphene nanobelt (n-gnrs), performing amination on a fullerene-palladium-platinum nanoparticle (n-C60 / pdpt) composite material and a staphylococcus aureus a protein (spa), and performing layer-by-layer self-assembling so as to immobilize a pcsk9 antibody (ab1); mixing the synthesized nanoparticle-poly-methylene blue (pt-pmb) with the pcsk9 antibody (ab2), and preparing a nano beacon, thereby obtaining the electrochemical immunosensor for pcsk9 protein detection. The electrochemical immunosensor has the advantages of being high in sensitivity, good in specificity and rapid and convenient in detection. A novel method for pcsk9 protein detection is provided, and useful information is provided for clinical prediction and diagnosis on cardiovascular diseases.
Owner:CHONGQING MEDICAL UNIVERSITY
Methods of lowering proprotein conversate subtilisin/kexin type 9 (PCSK9)
InactiveUS20140093513A1Convenient treatmentReduce plasma cholesterol levelBiocideSenses disorderSubtilisinsProprotein Convertase Inhibitors
The invention relates to new methods of modulating cholesterol by inhibiting proprotein convertase subtilisin / kexin type 9 (PCSK9) with fatty acid derivatives; and new methods for treating or preventing a metabolic disease comprising the administration of an effective amount of a fatty acid derivative. The present invention is also directed to fatty acid bioative derivatives and their use in the treatment of metabolic diseases.
Owner:CATABASIS PHARMA
Antagonists of pcsk9
Antagonists of human proprotein convertase subtilisin-kexin type 9 ('PCSK9') are disclosed. The disclosed antagonists are effective in the inhibition of PCSK9 function and, accordingly, present desirable antagonists for the use in the treatment of conditions associated with PCSK9 activity. The present invention also discloses nucleic acid encoding said antagonists, vectors, host cells, and compositions comprising the antagonists. Methods of making PCSK9-specific antagonists as well as methods of using the antagonists for inhibiting or antagonizing PCSK9 function are also disclosed and form important additional aspects of the present disclosure.
Owner:SCHERING AG
Methods for inhibiting atherosclerosis by administering an inhibitor of pcsk9
ActiveUS20160115246A1Reduces atherosclerotic plaque formationReduce formationOrganic active ingredientsAntibody ingredientsKexinAntigen binding
The present invention provides methods and compositions for inhibiting atherosclerotic plaque formation in a subject. In certain embodiments, the methods of the present invention comprise selecting a subject who has, or is at risk of developing, atherosclerosis, and administering to the subject a pharmaceutical composition comprising a proprotein convertase subtilisin / kexin type 9 (PCSK9) inhibitor. In certain embodiments, the PCSK9 inhibitor is an anti-PCSK9 antibody, or antigen binding protein.
Owner:SANOFI BIOTECH SAS +1
Methods of reducing a viral infection and kits therefore
InactiveUS20090104209A1Lower Level RequirementsHigh activityBiocideCompound screeningSubtilisinKexin
A method for treating and / or preventing a proprotein convertase subtilisin / kexin type 9 preproprotein (PCSK9)-susceptible viral infection comprising increasing a PCSK9 activity and / or expression in a biological system infected by the virus, whereby the increased PCSK9 activity and / or expression treats and / or prevents the viral infection in the biological system. Methods of classifying subjects, methods of screening and kits therefore.
Owner:INSTITUT NATIONAL DE LA RECHERCHE SCIENTIFIQUE +1
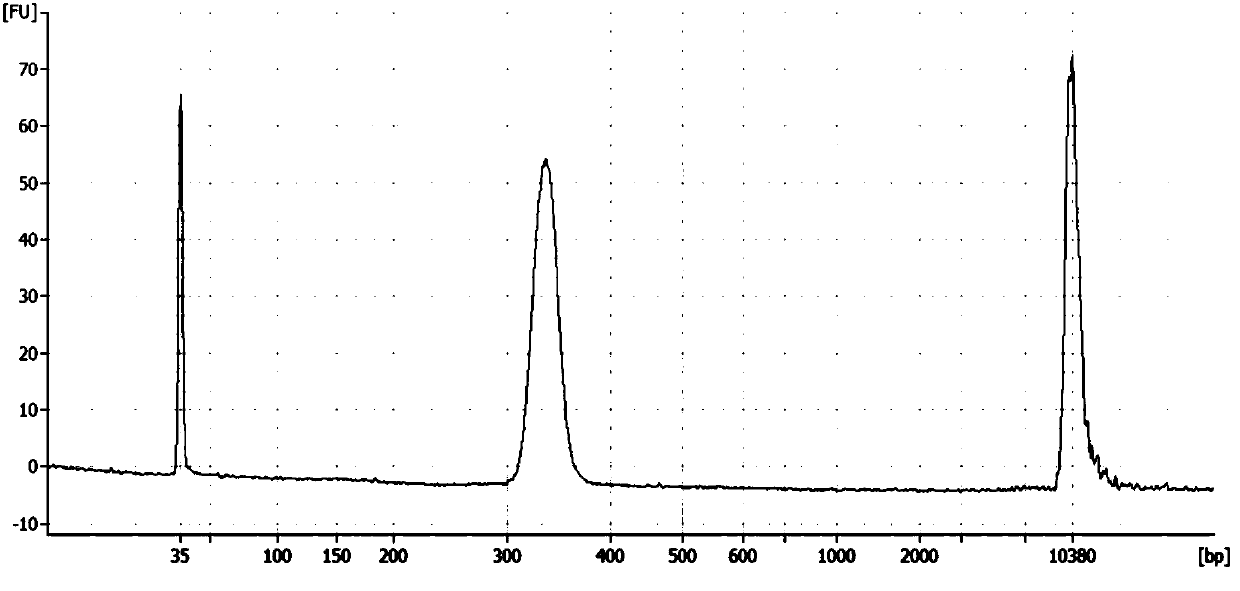
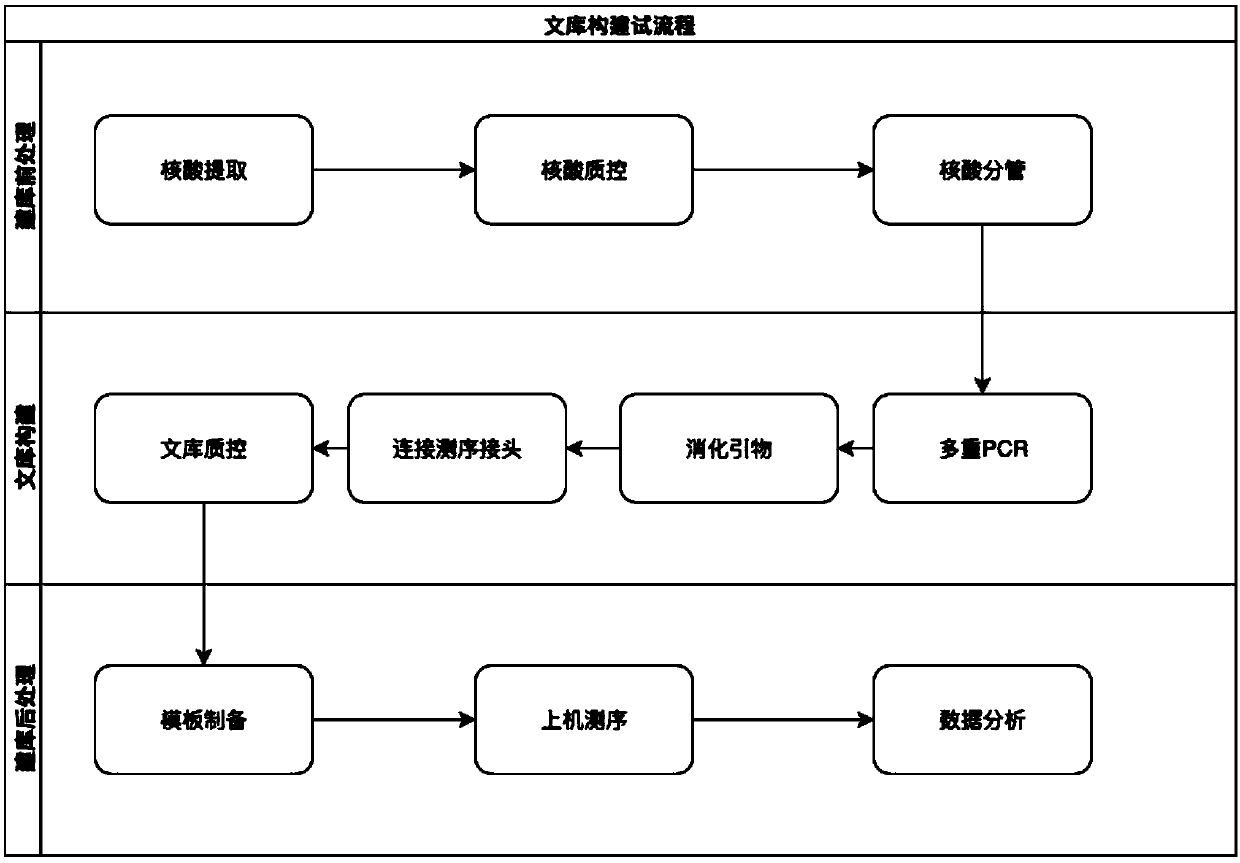
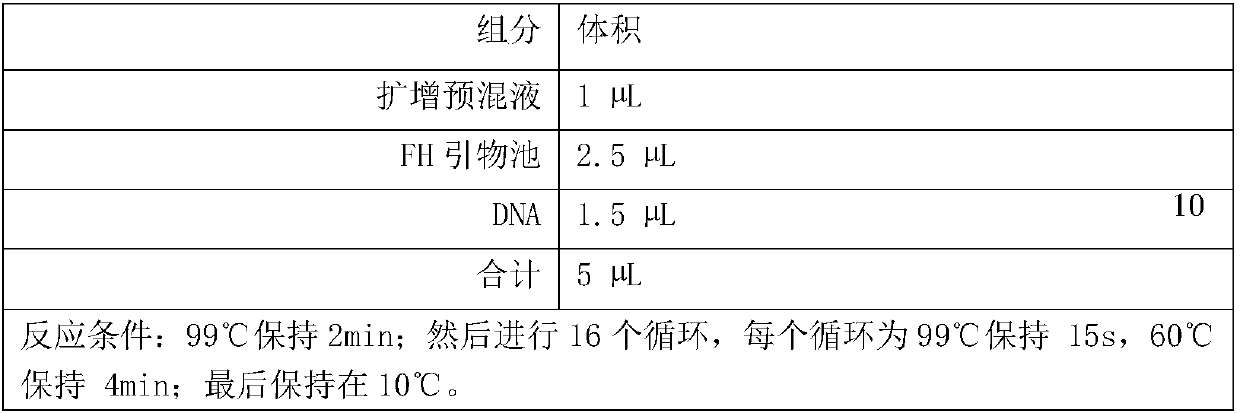
Construction method of gene detection library of familial hypercholesterolemia and gene detection kit
ActiveCN109517884AHigh precisionEasy to operateMicrobiological testing/measurementLibrary creationSubtilisinKexin
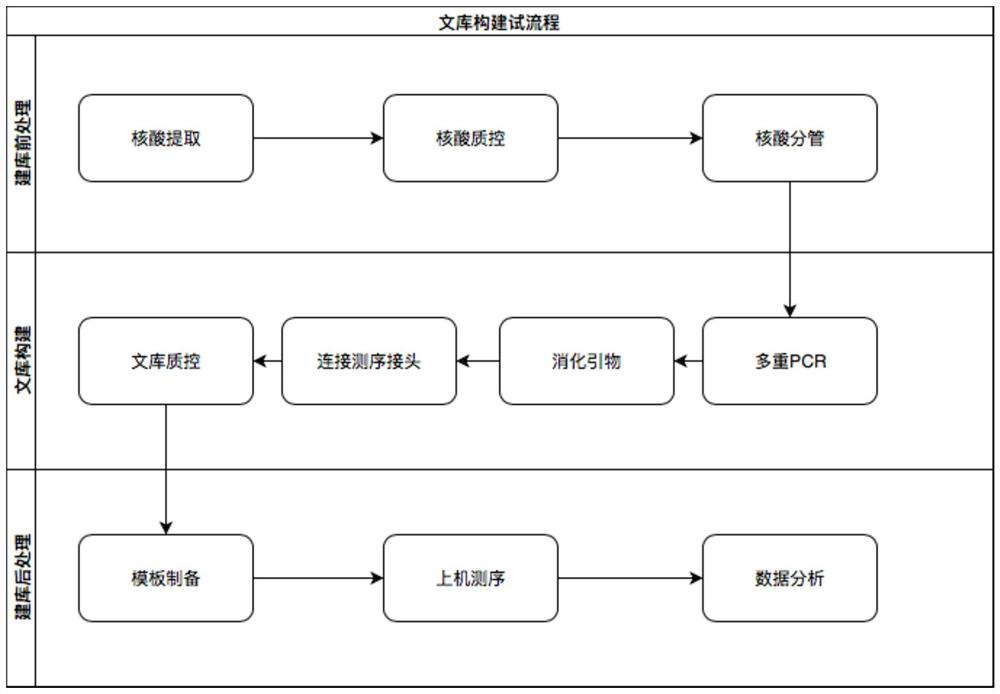
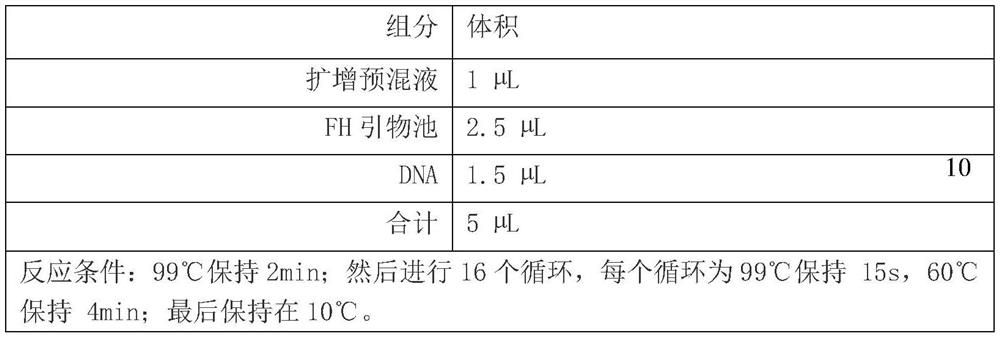
The invention discloses a construction method of a gene detection library of familial hypercholesterolemia and a gene detection kit and relates to gene mutation of LDLR (Low-Density Lipoprotein Receptor), APOB (Apolipoprotein B) and PCSK9 (Proprotein Convertase Subtilisin / Kexin type 9). In order to ensure that all target regions (including an entire coding region and an alternative splicing regionof a gene to be detected) are covered and prevent a primer dimer or a short segment from being formed between primers of adjacent amplicons, a PCR (Polymerase Chain Reaction) amplification primer isdivided into two independent primer pools; then primer sequence digestion, sequencing connector connection, library purification and quality detection are carried out; finally, the detection library is constructed. The method has the advantages that library establishment steps are simple and rapid, the cost is effectively reduced, the working amount is reduced, variation types are comprehensive, the detection accuracy reaches 100 percent and the flux is high.
Owner:ANNGEEN BIOTECHNOLOGY CO LTD
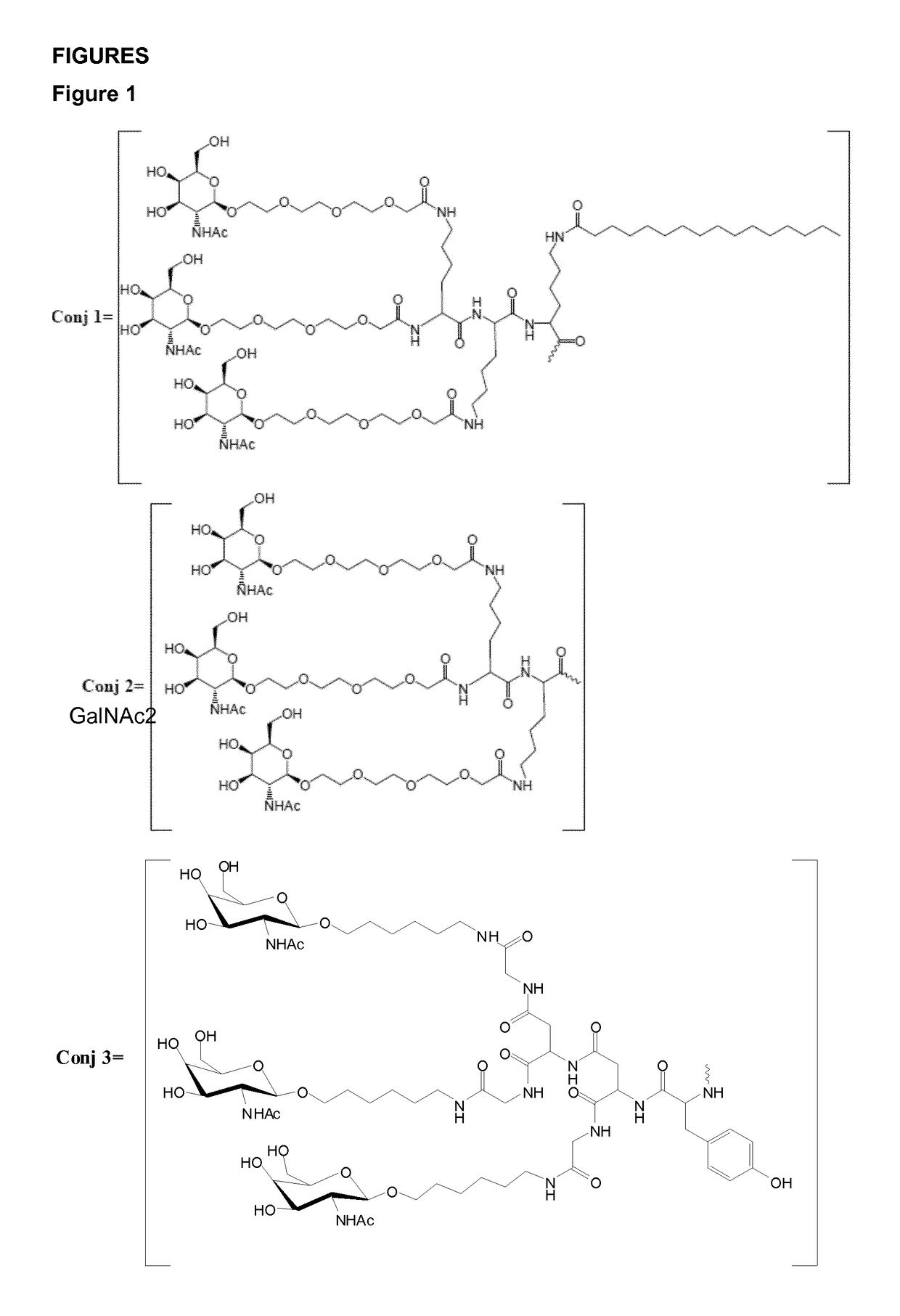
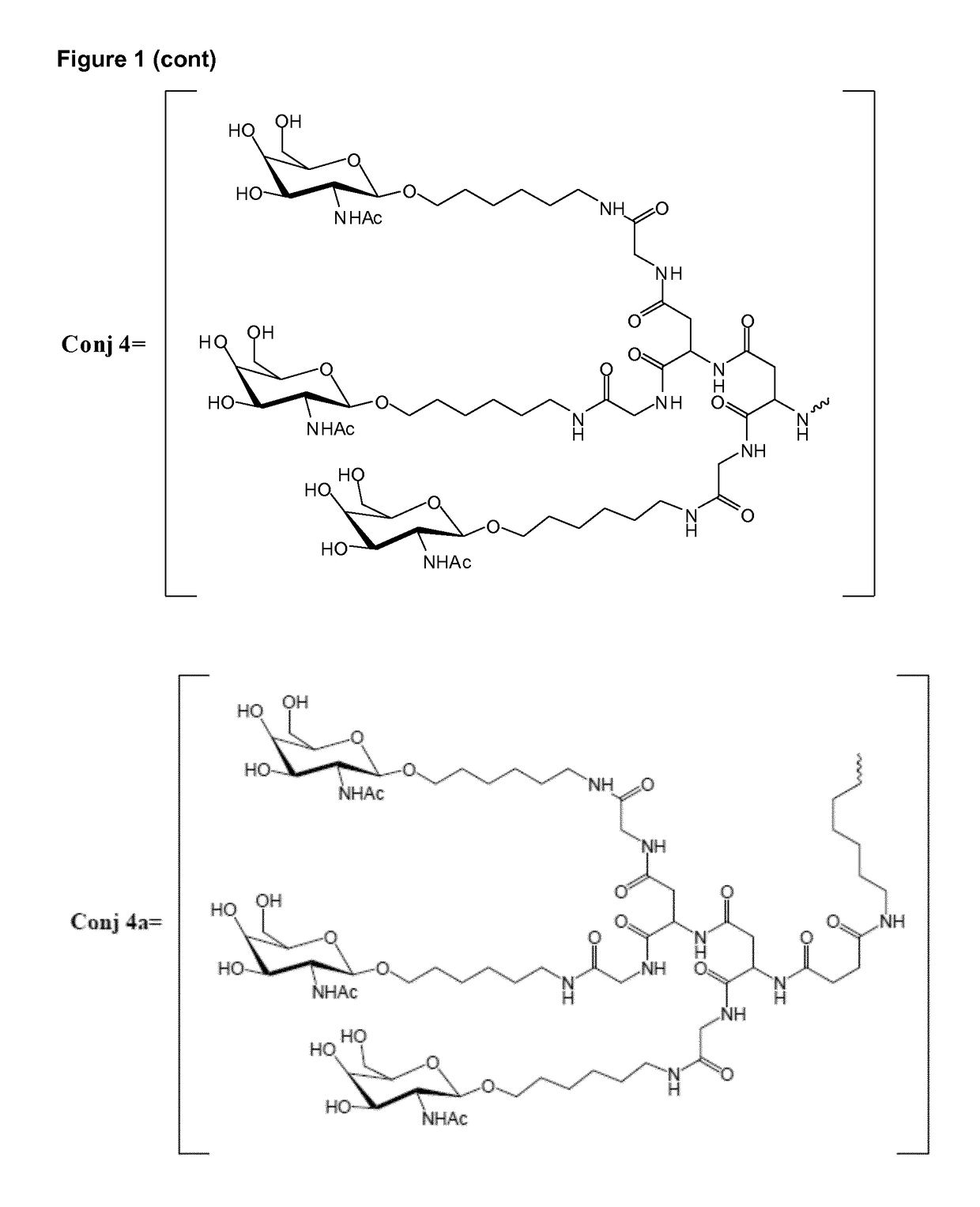
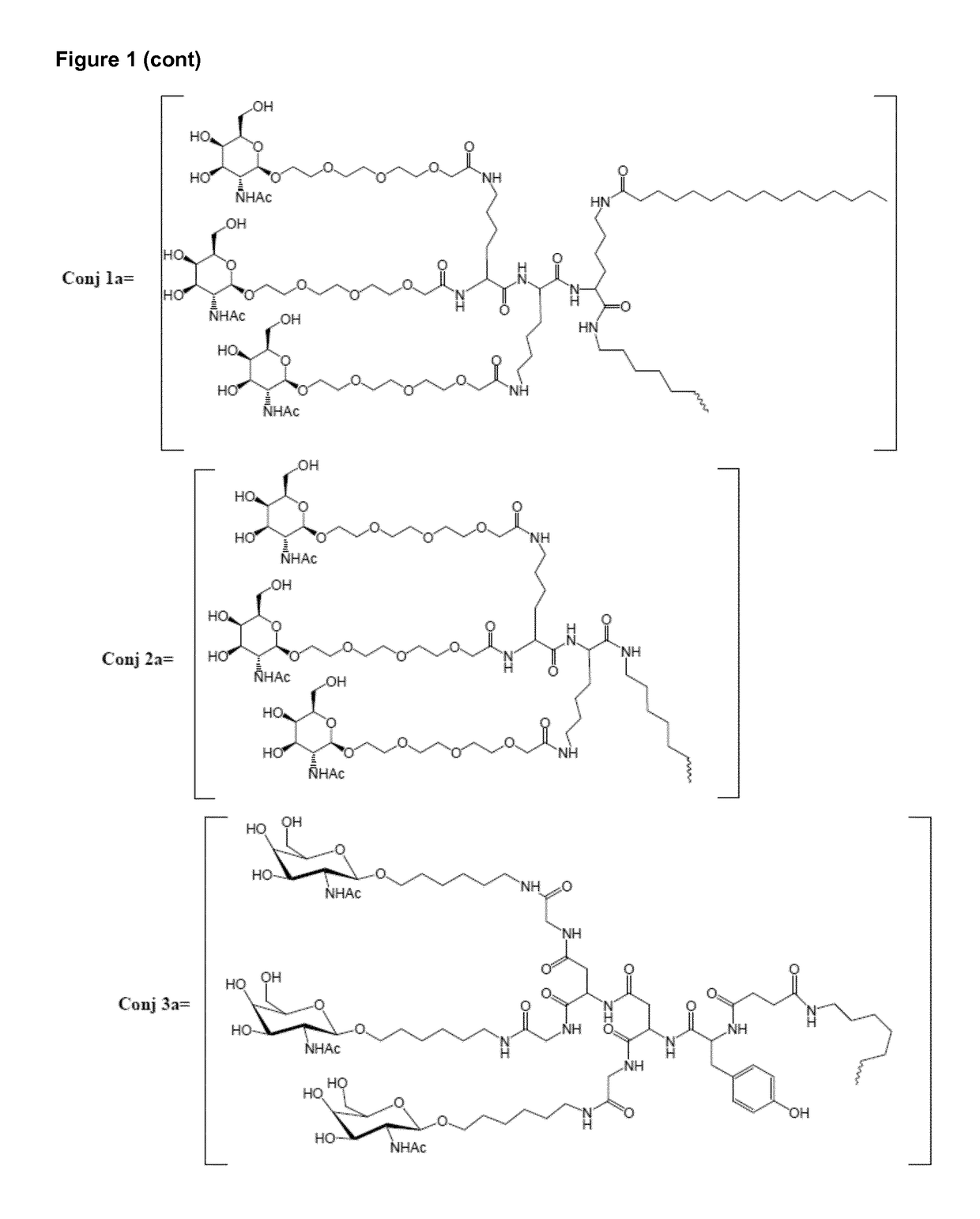
Oligonucleotide conjugates
ActiveUS9879265B2Reduce Toxicity RiskReduce nephrotoxicityOrganic active ingredientsSugar derivativesMedical disorderSubtilisins
The present invention relates to oligomeric compounds and conjugates thereof that target Proprotein Convertase Subtilisin / Kexin type 9 (PCSK9) PCSK9 mRNA in a cell, leading to reduced expression of PCSK9. Reduction of PCSK9 expression is beneficial for a range of medical disorders, such as hypercholesterolemia and related disorders.
Owner:ROCHE INNOVATION CENT COPENHAGEN
Anti-pcsk9 antibodies with ph-dependent binding characteristics
The present invention provides antibodies and antigen-binding fragments thereof that specifically bind proprotein convertase subtilisin / kexin-9 (PCSK9) with greater affinity at neutral pH than at acidic pH. The antibodies of the invention may possess one or more amino acid changes as compared to antibodies that do not exhibit pH-dependent binding properties. For example, the present invention includes anti-PCSK9 antibodies which possess one or more histidine substitutions in one or more complementarity determining regions. The antibodies of the invention, with pH-dependent binding properties, remain in circulation and exhibit cholesterol lowering activity for prolonged periods of time in animal subjects as compared to anti-PCSK9 antibodies that do not exhibit pH-dependent binding properties. The antibodies of the invention are therefore useful for treating diseases and disorders related to elevated HDL cholesterol, wherein the antibodies of the invention can be administered to a patient at a lower dose and / or with less frequent dosing as compared to antibodies that do not exhibit pH-dependent binding properties.
Owner:REGENERON PHARM INC
PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) inhibitor in preparing medicine for treating T-cell medicated inflammatory-immune diseases
InactiveCN108066763AImprove side effectsLittle side effectsMetabolism disorderAntipyreticImmunologic disordersDisease
The invention belongs to the technical field of medicine biologics, relates to functions and mechanisms of PCSK9 (Proprotein Convertase Subtilisin / Kexin 9) in treating T-cell medicated inflammatory-immune diseases, application of a PCSK9 inhibitor in preparing a medicine for treating T-cell medicated inflammatory-immune diseases, and in particular relates to application of a PCSK9 small-molecule interference RNA or PCSK9 small-molecule inhibitor in preparing medicines for preparation systems or external skin application in treating psoriasis, atopic dermatitis or urticaria. Psoriasis is adopted as a testing bed for inflammatory-immune disease research, results show that the PCSK9 small-molecule inhibitor or small-molecule interference RNA has treatment effects which are remarkably prior tothose of PCSK9 monoclonal antibodies in treating inflammation such as psoriasis. The PCSK9 small-molecule interference RNA or PCSK9 small-molecule inhibitor can be further developed into novel medicines for treating inflammatory-immune diseases such as psoriasis, and is small in side effect, low in cost and remarkable in treatment effect.
Owner:陈敏
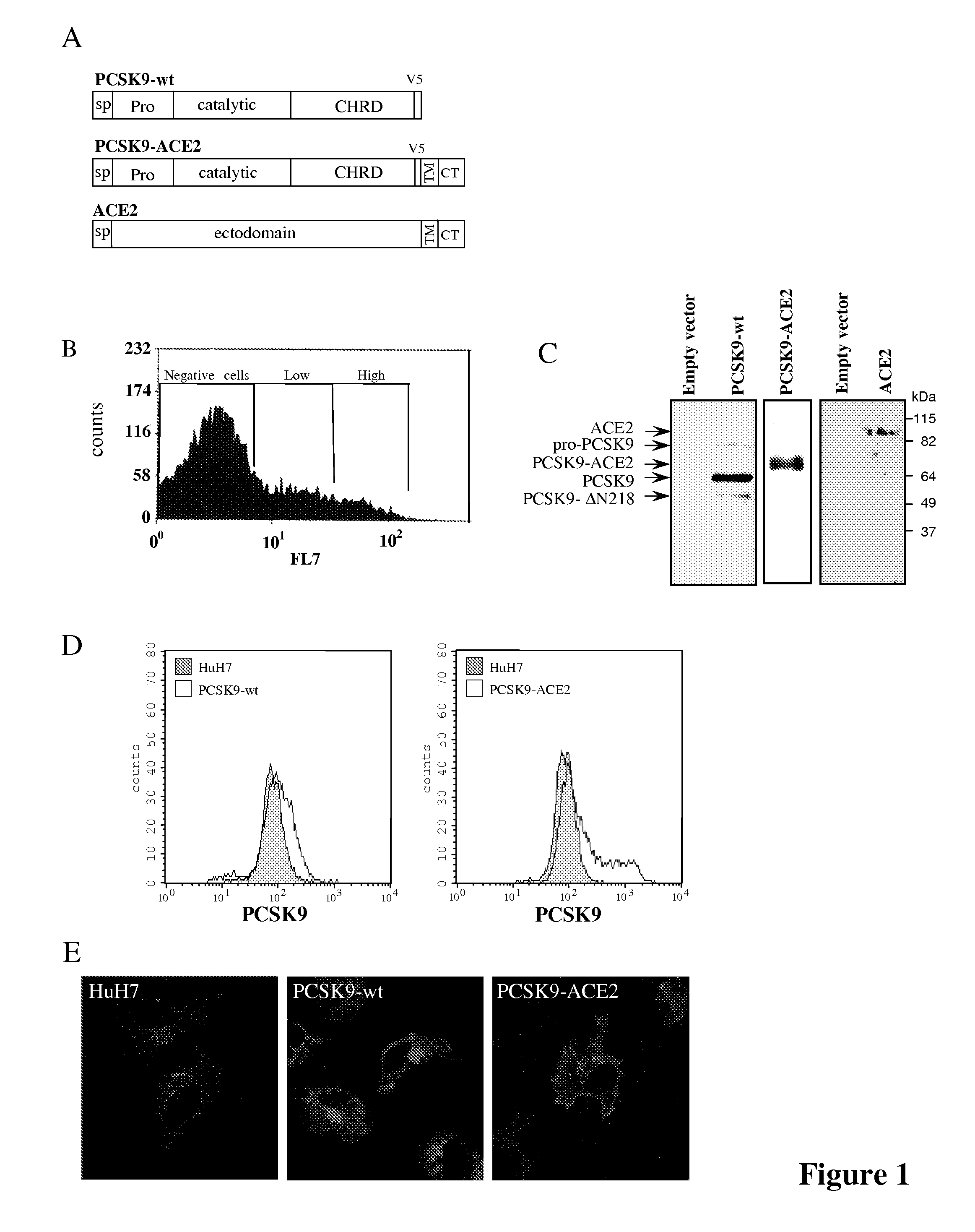
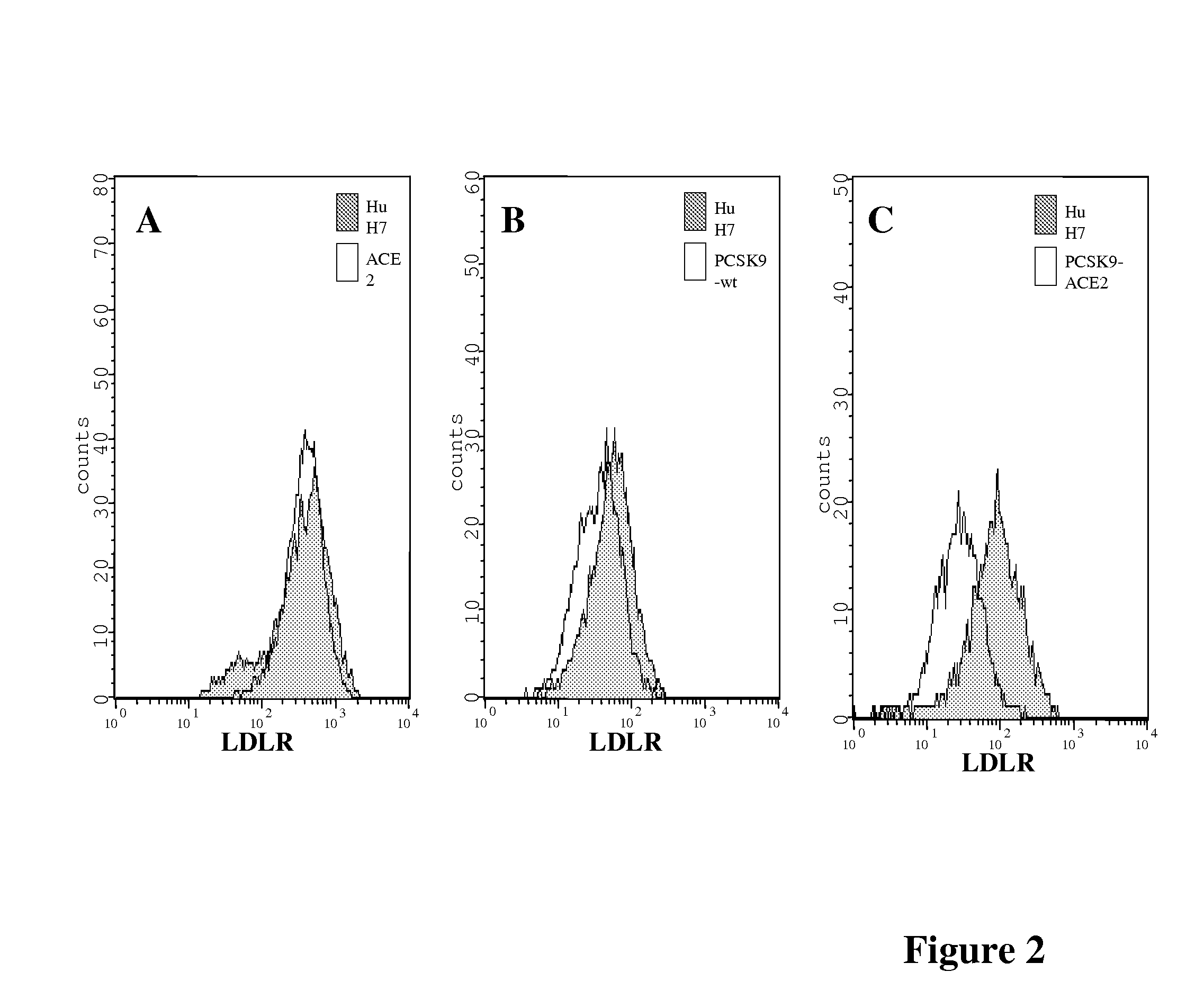
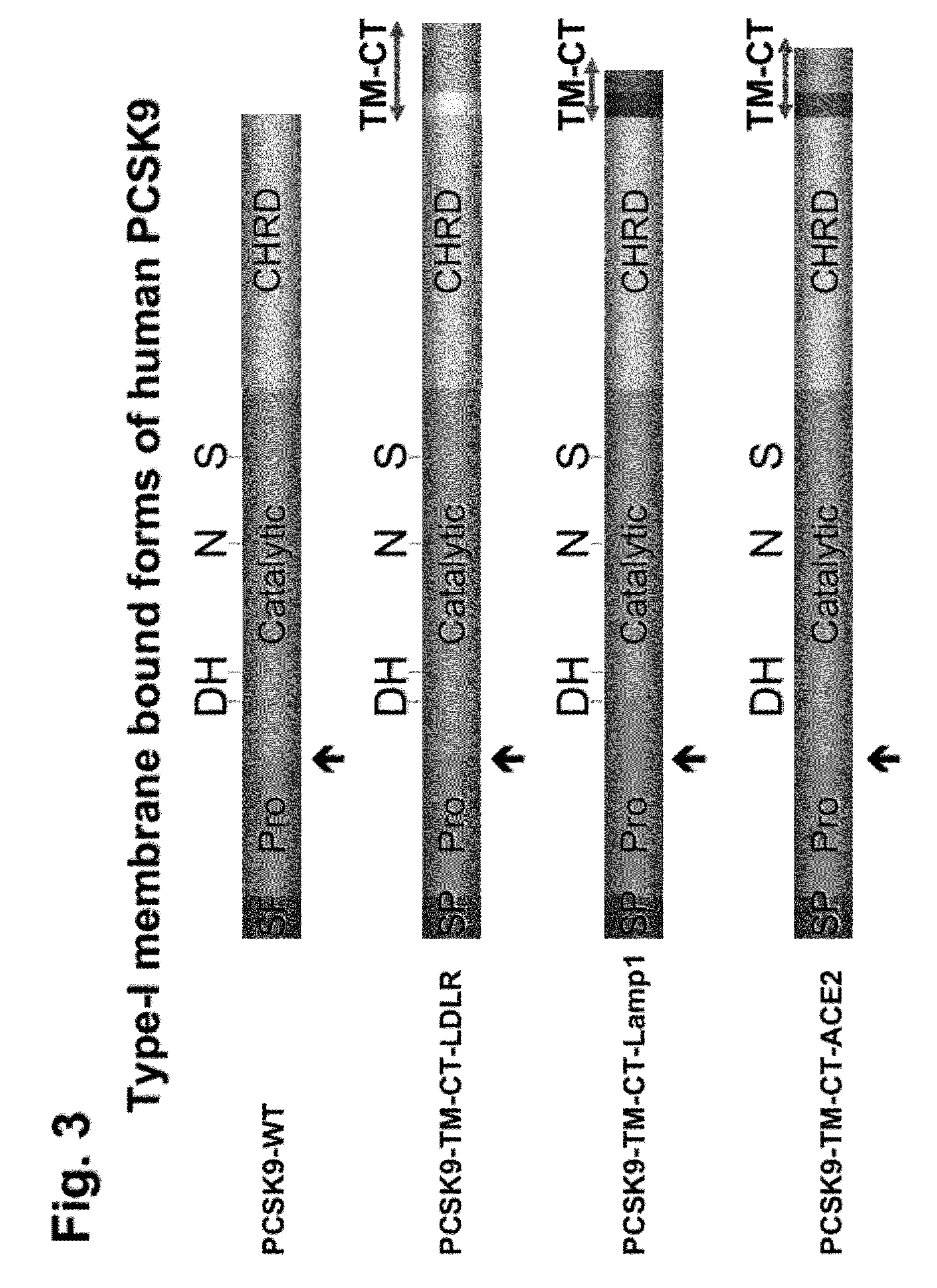
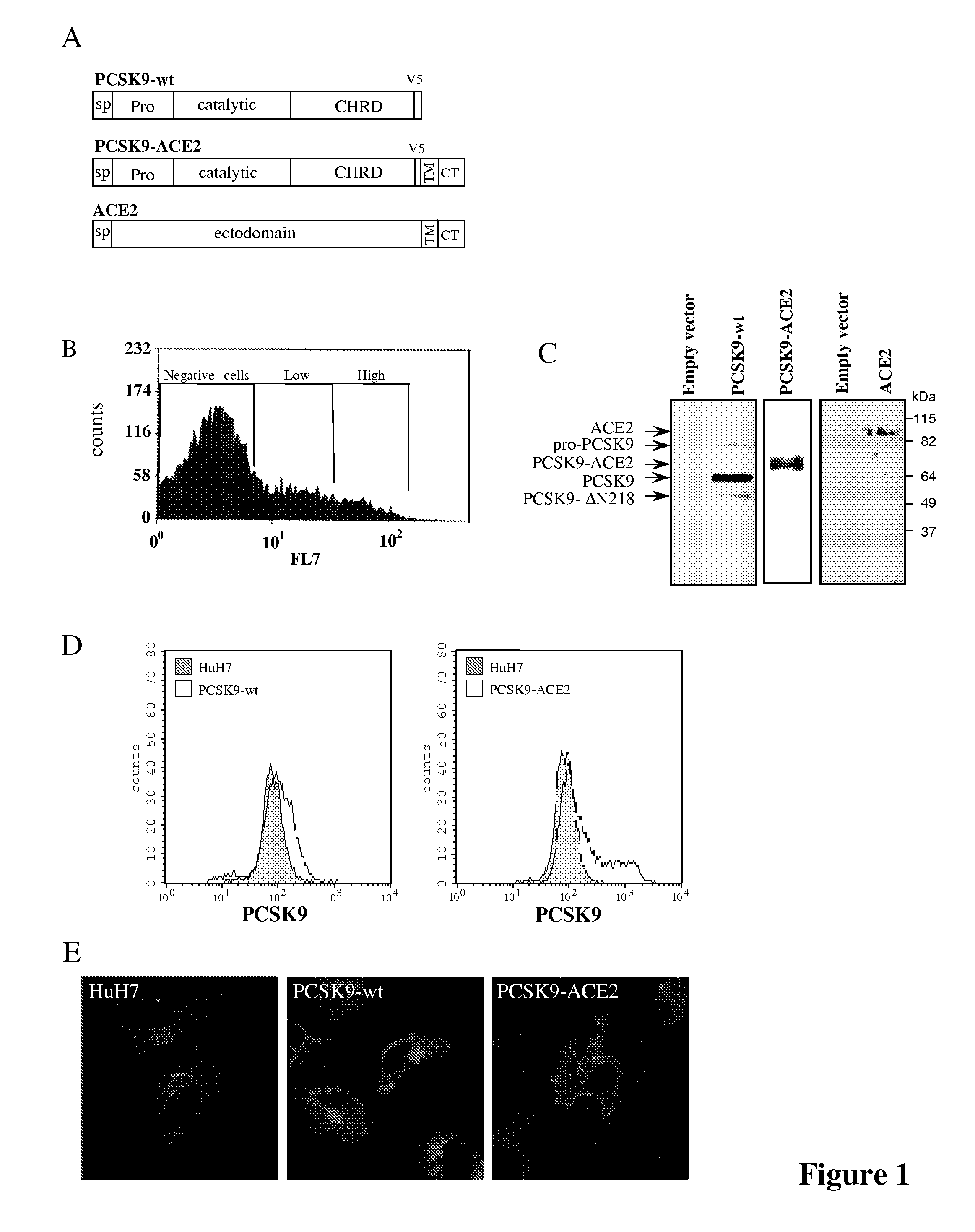
Chimeric pcsk9 proteins, cells comprising same, and assays using same
ActiveUS20110003315A1High level of sensitivityStrong specificityPolypeptide with localisation/targeting motifPeptide/protein ingredientsSubtilisinKexin
A chimera protein comprising in the following order: a signal peptide, a proprotein convertase subtilisin / kexin type 9 preproprotein (PCSK9) sequence consisting of amino acid residues at positions 35 to 696 of SEQ ID NO: 38, a transmembrane domain and a cytosolic domain, wherein said cytosolic (CT) domain comprises a sequence able to recycle the protein from the cellular membrane to endosomes.
Owner:ADAERATA
Chimeric pcsk9 proteins, cells comprising same, and assays using same
ActiveUS20120237945A1Promote degradationHigh expressionPolypeptide with localisation/targeting motifPeptide/protein ingredientsKexinSubtilisin
A chimera protein comprising in the following order: a signal peptide, a proprotein convertase subtilisin / kexin type 9 preproprotein (PCSK9) sequence consisting of amino acid residues at positions 35 to 696 of SEQ ID NO: 38, a transmembrane domain and a cytosolic domain, wherein said cytosolic (CT) domain comprises a sequence able to recycle the protein from the cellular membrane to endosomes.
Owner:ADAERATA
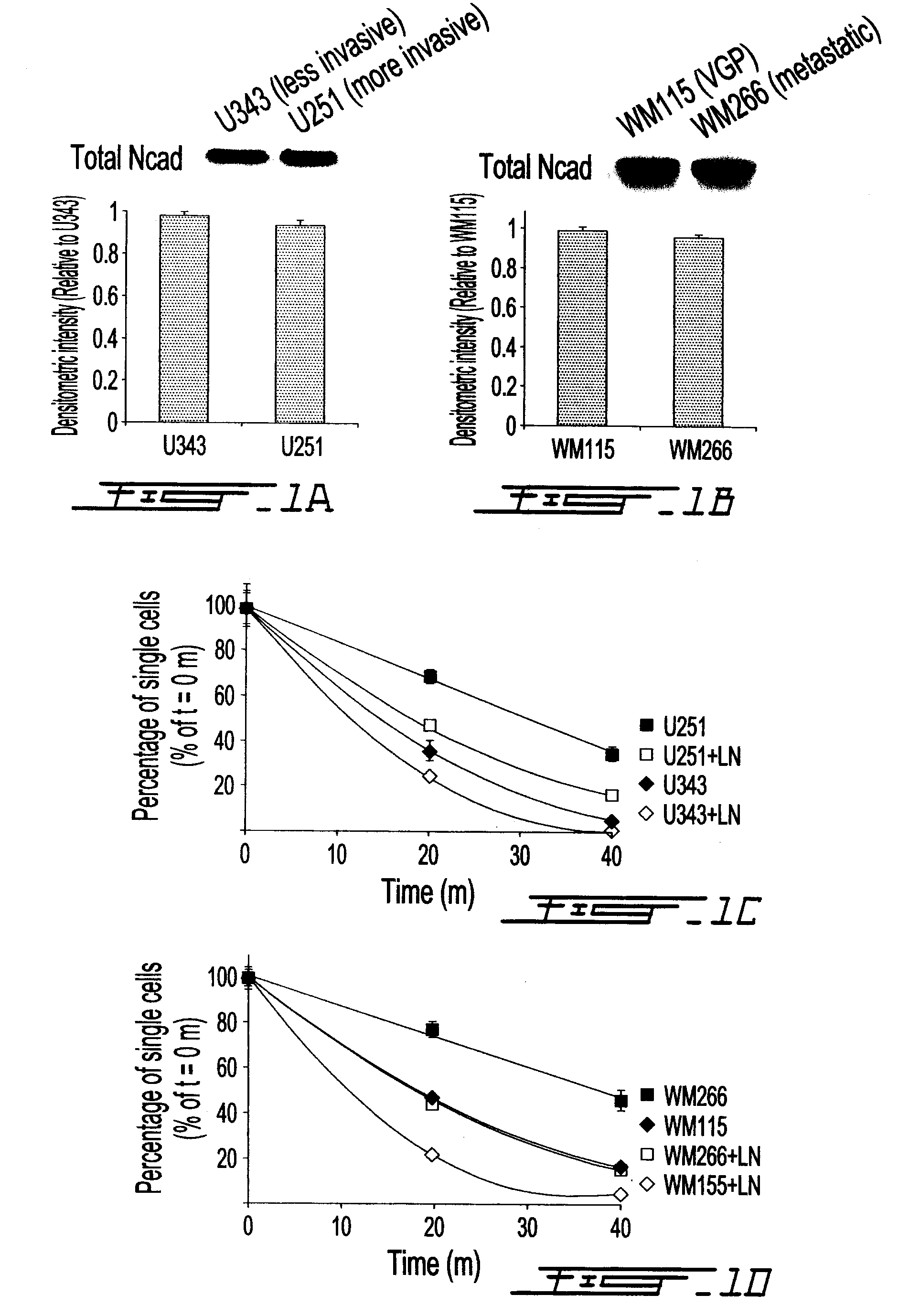
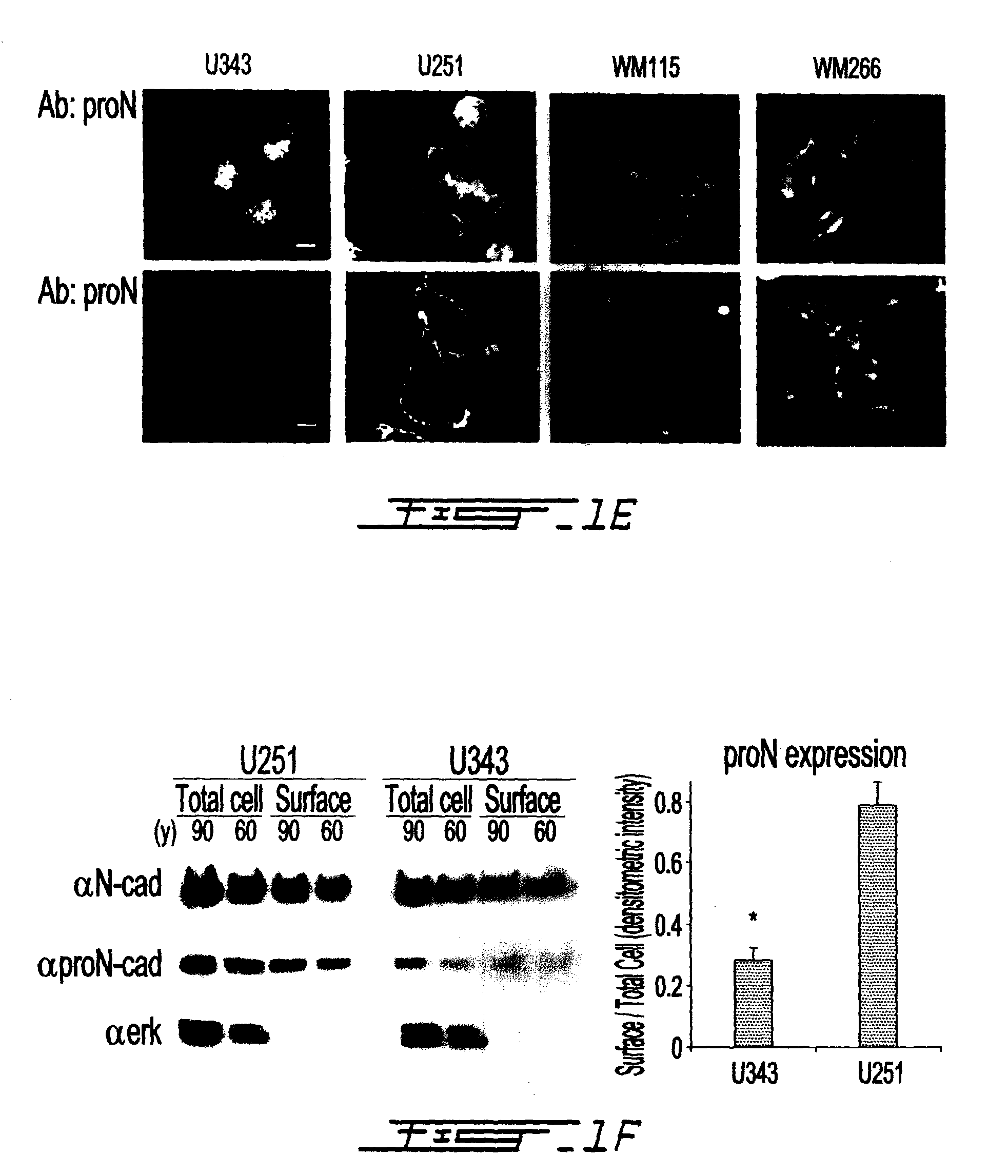
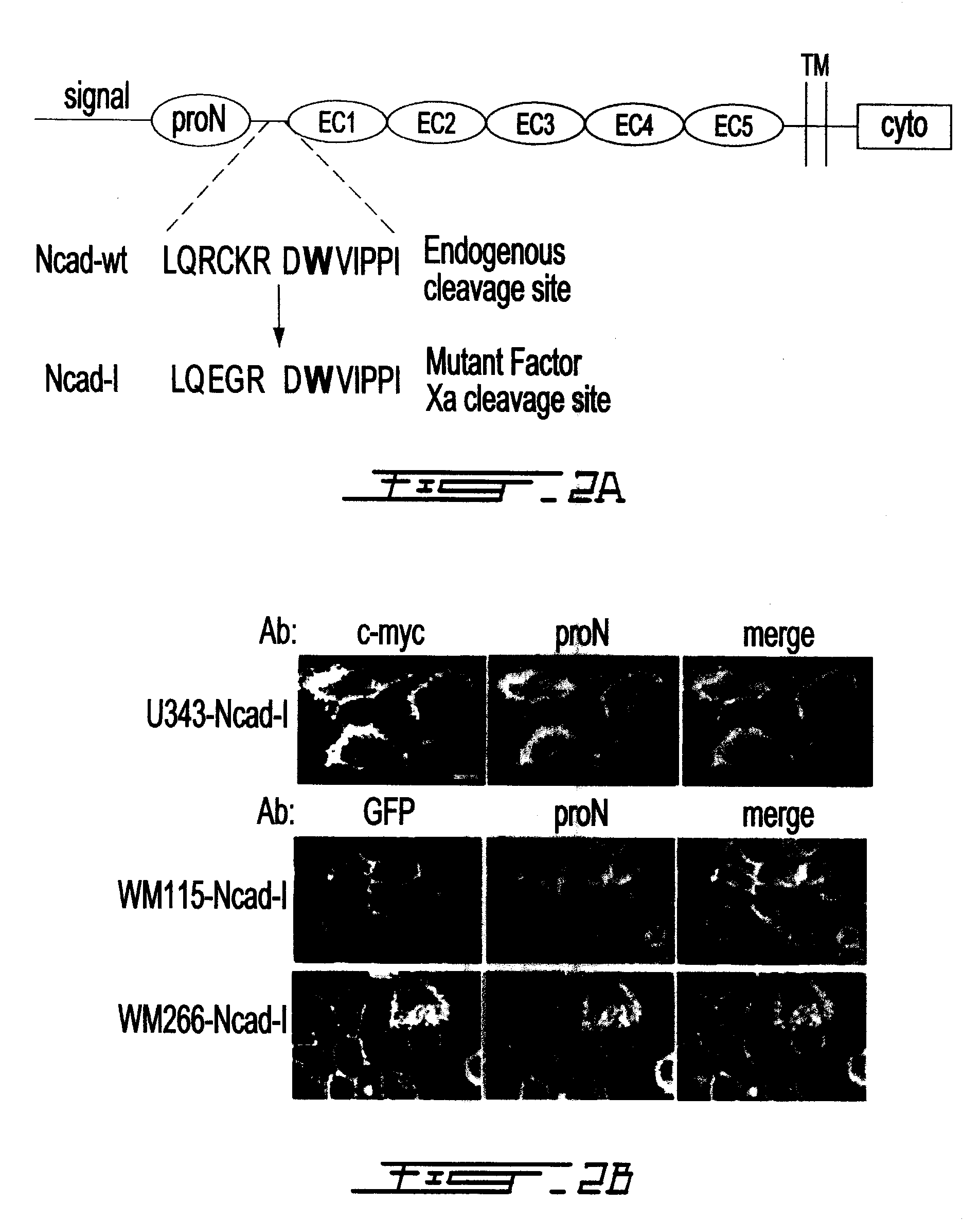
Altered n-cadherin processing in tumor cells by furin and proprotein convertase 5a (PC5A)
InactiveUS20100310451A1Increase productionReduce productionOrganic active ingredientsIn-vivo radioactive preparationsLymphatic SpreadCancer cell
The present invention relates to a method for diagnosis and / or prognosis of cancer and for monitoring the progression of cancer and / or the therapeutic efficacy of an anti-cancer treatment in a subject by determining the molecular form of cadherin at the cell surface of cancer cells in the subject. The invention also relates to a method for preventing, inhibiting or treating cancer or its metastasis in a subject by increasing the adhesive forms of cadherin and / or decreasing the non-adhesive forms of cadherin at the cell surface. The invention also relates to a method step of determining the expression level of furin and proprotein convertase 5A (PC5A).
Owner:MCGILL UNIV +2
PCSK9 (Proprotein Convertase Subtilisin Kexin Type 9) resistant monoclonal antibody
ActiveCN107698680ABinding blockIncrease intakeMetabolism disorderMammal material medical ingredientsKexinPhage antibodies
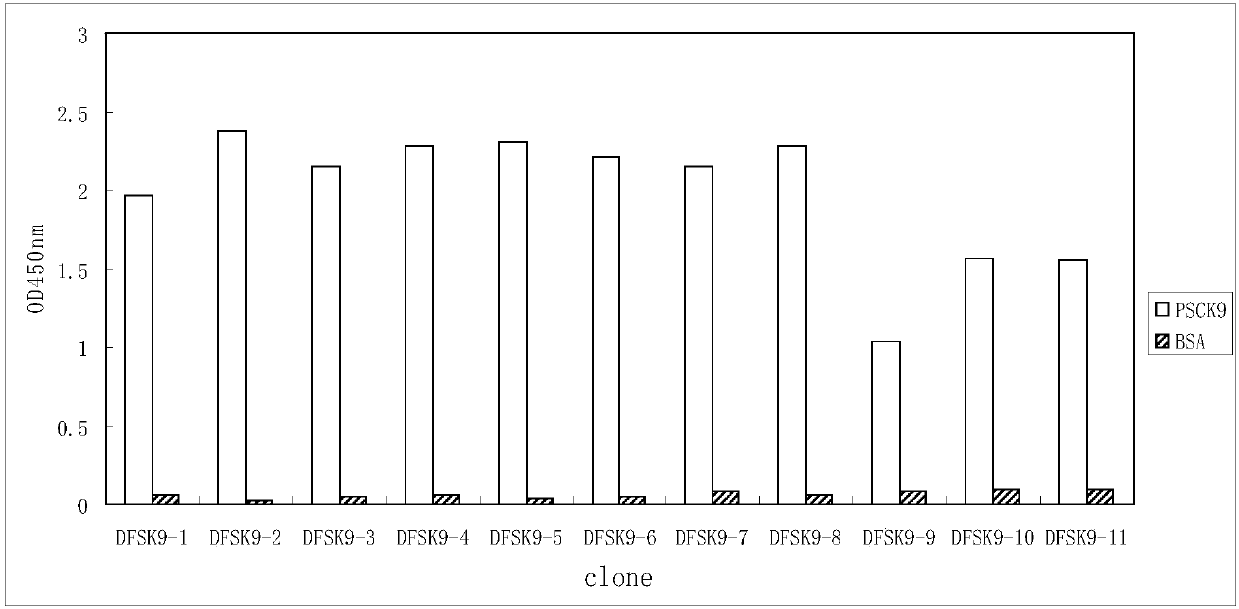
The invention relates to the technical field of antibody engineering and in particular discloses a PCSK9 (Proprotein Convertase Subtilisin Kexin Type 9) resistant monoclonal antibody. The monoclonal antibody disclosed by the invention comprises an amino acid sequence coding an antibody variable region and a CDR region. The invention further discloses an acquiring method and application of the monoclonal antibody. The method comprises the following steps: screening a PCSK9 resistant monoclonal antibody from a phage antibody library, performing affinity maturation through a method for constructing the phage antibody library by virtue of strand displacement, performing mutant library-construction screening on light-chain CDR1, 2 and 3 regions of the monoclonal antibody obtained by preliminaryscreening, selecting a monoclonal antibody with high affinity, performing mutant library-construction screening on heavy-chain CDR1, 2 and 3 regions of the monoclonal antibody, and finally screeningthe PCSK9 resistant monoclonal antibody with high affinity. The PCSK9 resistant monoclonal antibody obtained in the invention has excellent affinity to PCSK9, is capable of inhibiting binding betweenthe PCSK9 and ligands thereof, and can be used for treating dyslipidemia, cardiovascular and cerebrovascular diseases and thrombosis-obstructive diseases.
Owner:BEIJING DONGFANG BIOTECH
Dosing regimens for use with pcsk9 inhibitors
InactiveUS20200024364A1Reducing LDL-CGain is not constantMetabolism disorderAntibody ingredientsSubtilisinDosing regimen
The present invention provides methods for treating a PCSK9-mediated disease or a PCSK9-mediated condition. Specifically, the invention relates to methods comprising the administration of a proprotein convertase subtilisin / kexin type 9 (PCSK9) antibody or antigen binding protein, in the absence of a statin, to a subject in need thereof.
Owner:REGENERON PHARM INC
Delivery system and biological preparation of PCSK9 (Proprotein Convertase subtilisin/kexin type 9) inhibitor hypolipidemic drug
ActiveCN108342387ASafe and efficient deliveryAvoid security issuesOrganic active ingredientsMetabolism disorderSubtilisinKexin
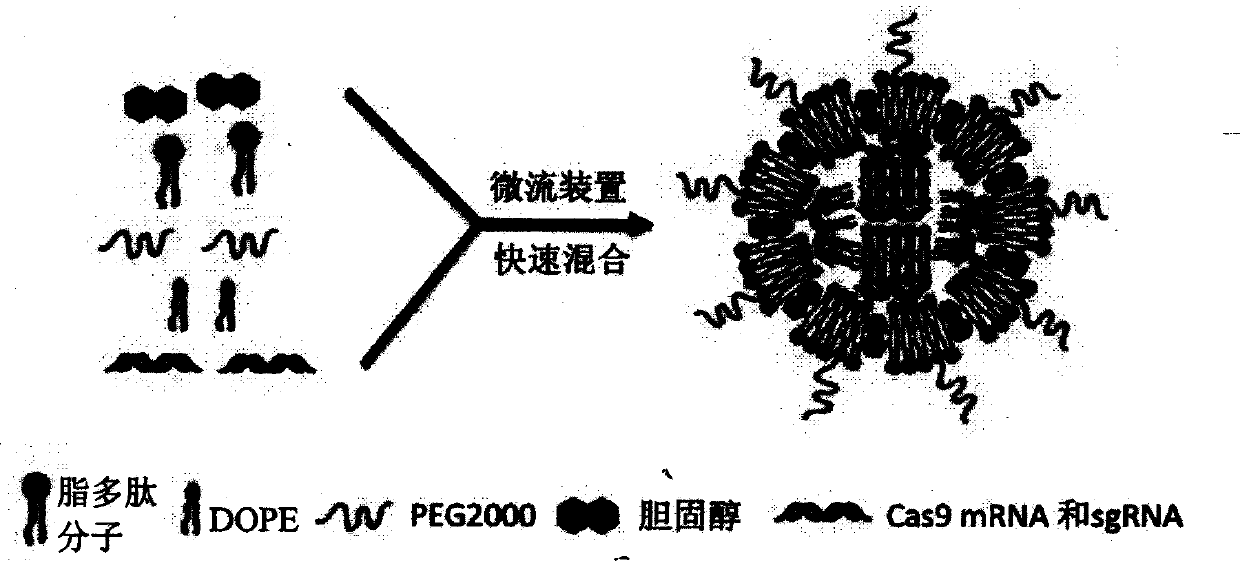
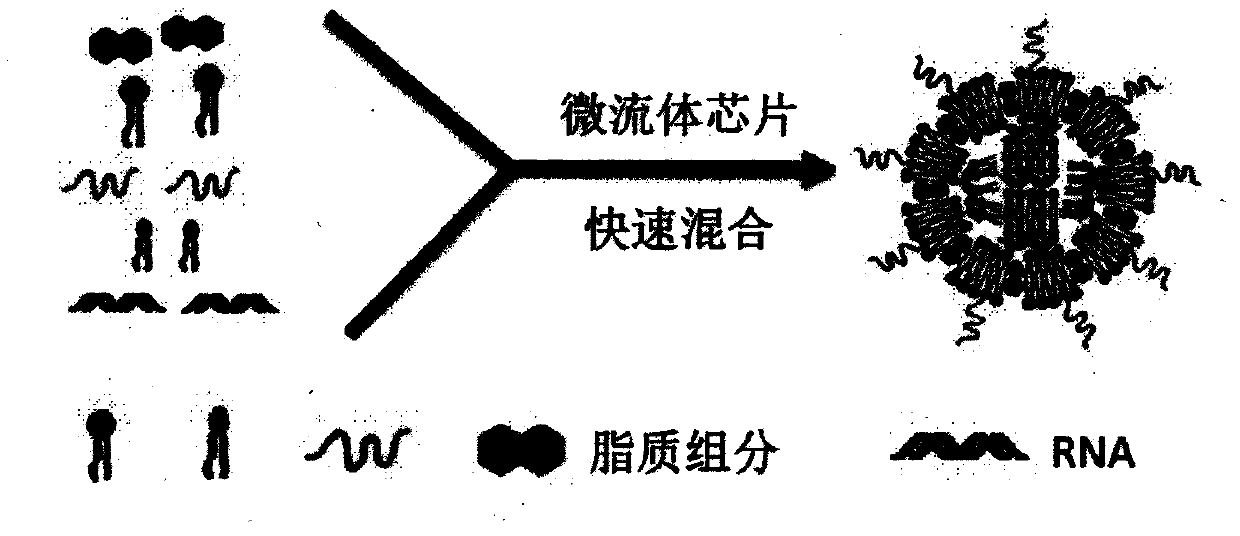
The present invention discloses a lipid nanoparticle comprising a lipopeptide molecule, dioleoyl phosphoethanolamine, cholesterol, and polyethylene glycol, and the lipid nanoparticle encapsulates Cas9mRNA and a sgRNA molecule in a gene conserved region of target mouse and human Proprotein Convertase subtilisin / kexin type 9 ( PCSK9). The present invention also discloses a preparation method and use of the lipid nanoparticle, and a pharmaceutical composition and formulation comprising the lipid nanoparticle. The lipid nanoparticle and the biological preparation can be used as a novel delivery system for a PCSK9 inhibitor hypolipidemic drug.
Owner:谭旭
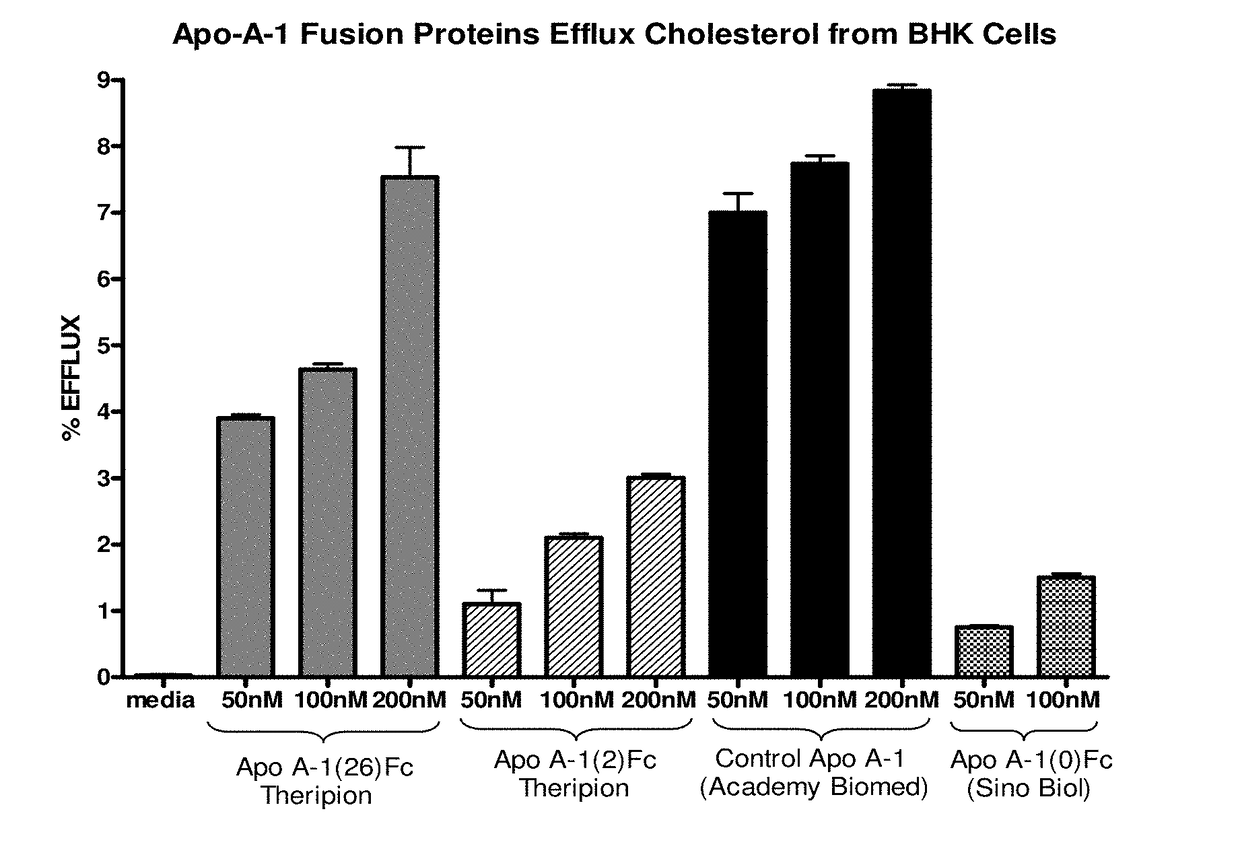
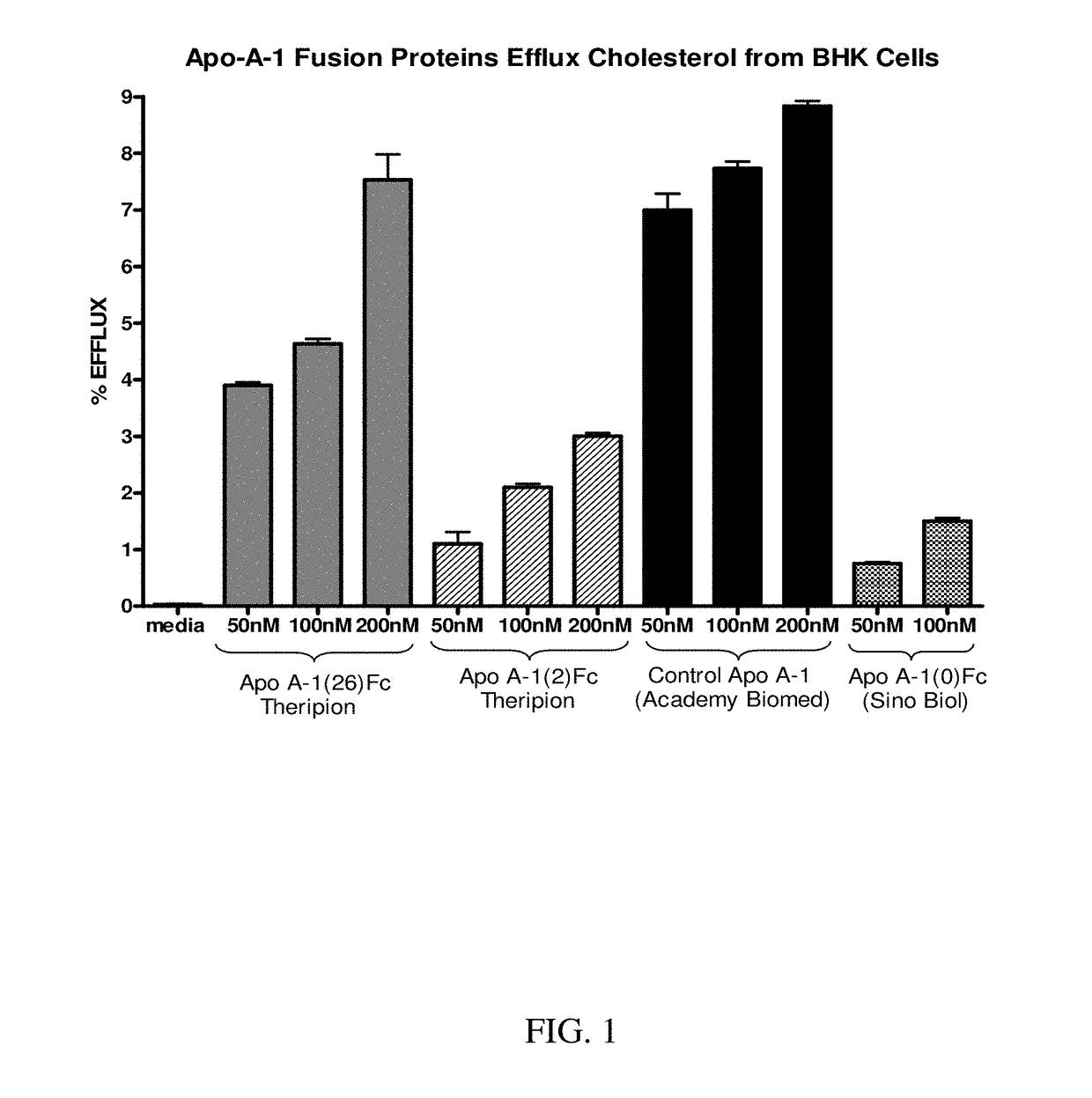
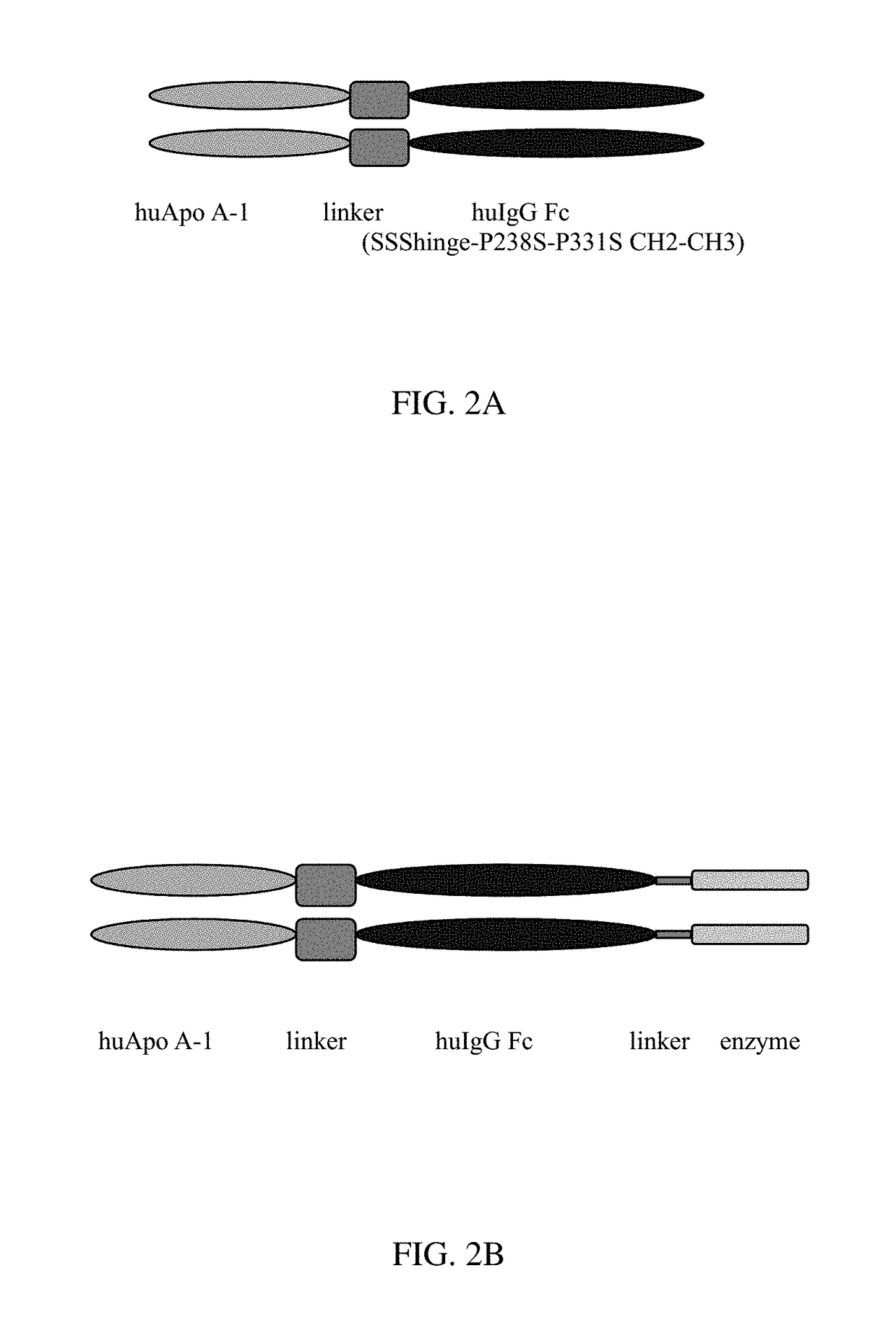
Apoa-1 fusion polypeptides and related compositions and methods
PendingUS20180201664A1Inhibit occurrenceAvoid symptomsAntibacterial agentsNervous disorderDimerSterol ester
Compositions and methods relating to ApoA-1 fusion polypeptides are disclosed. The fusion polypeptides include a first polypeptide segment corresponding to an ApoA-1 polypeptide or ApoA-1 mimetic, and may also include a dimerizing domain such as, e.g., an Fc region, which is typically linked carboxyl-terminal to the first polypeptide segment via a flexible linker. In some embodiments, the fusion polypeptide further includes a second polypeptide segment located carboxyl-terminal to the first polypeptide segment and which confers a second biological activity (e.g., an RNase, paraoxonase, platelet-activating factor acetylhydrolase, cholesterol ester transfer protein, lecithin-cholesterol acyltransferase, polypeptide that specifically binds to proprotein convertase subtilisin / kexin type 9, or polypeptide that specifically binds to amyloid beta). Also disclosed are dimeric proteins comprising first and second ApoA-1 fusion polypeptides as disclosed herein. The fusion polypeptides and dimeric proteins are useful in methods for therapy.
Owner:THERIPION INC
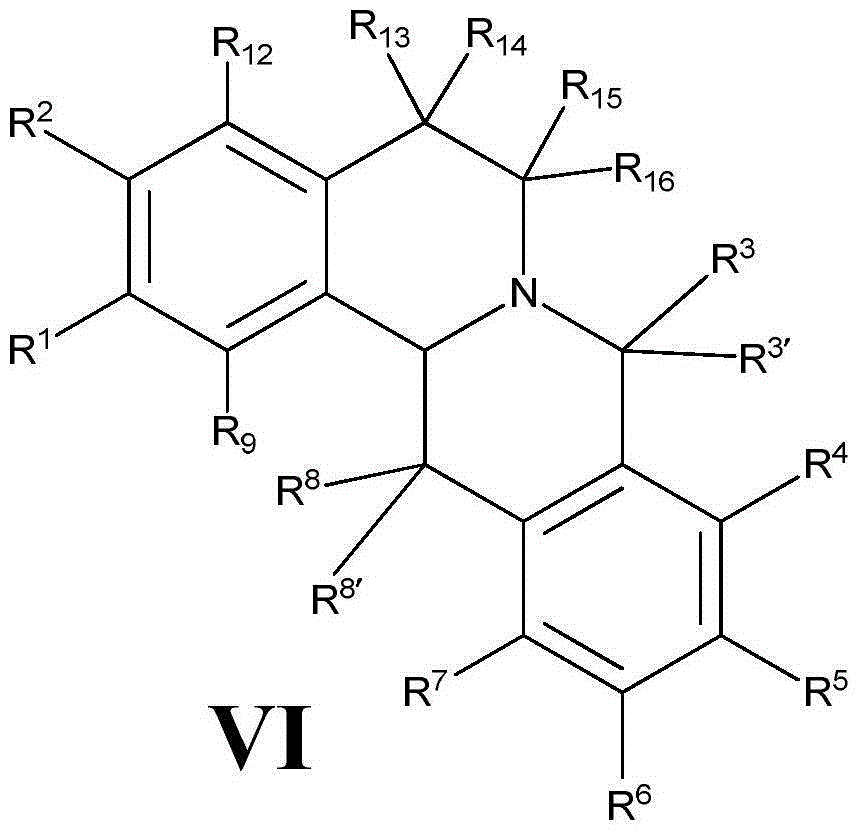
Tetrahydropalmatine derivative and application thereof
InactiveCN105481849AOrganic active ingredientsMetabolism disorderAcute hyperglycaemiaInsulin resistance
The invention relates to a tetrahydropalmatine derivative and application thereof, a compound as shown in a formula (VI) and a preparation method thereof, and an application of the compound in medicine. Specifically, the invention relates to a derivative of the compound as shown in the general formula (VI) and a preparation method thereof, and an application of the derivative as a therapeutic agent in prevention and treatment of hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, hepatic steatosis, diabetes type II, hyperglycemia, adiposis, or insulin resistance and metabolic syndrome. The compound disclosed by the invention also can reduce total cholesterol, low density lipoprotein (LDL)-cholesterol and triglycerides, and increases the expression of a liver LDL receptor and inhibits the expression of proprotein convertase subtilisin / kexin type 9 (PCSK9).
Owner:CHENGDU BESTCHIRALBIO LIMITED LIABILITY
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a one or more assays configured to detect a kidney injury marker selected from the group consisting of Stanniocalcin-1, Antithrombin-III, Toll-like receptor 2, Triiodothyronine (T3), Thyroxine (T4), Extracellular matrix protein 1, Coagulation factor XIII A chain, Coagulation factor XIII B chain, Interleukin-17F, Interleukin-22, Vitronectin, Progesterone, Estradiol, Growth / differentiation factor 15, and Proprotein convertase subtilisin / kexin type 9 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
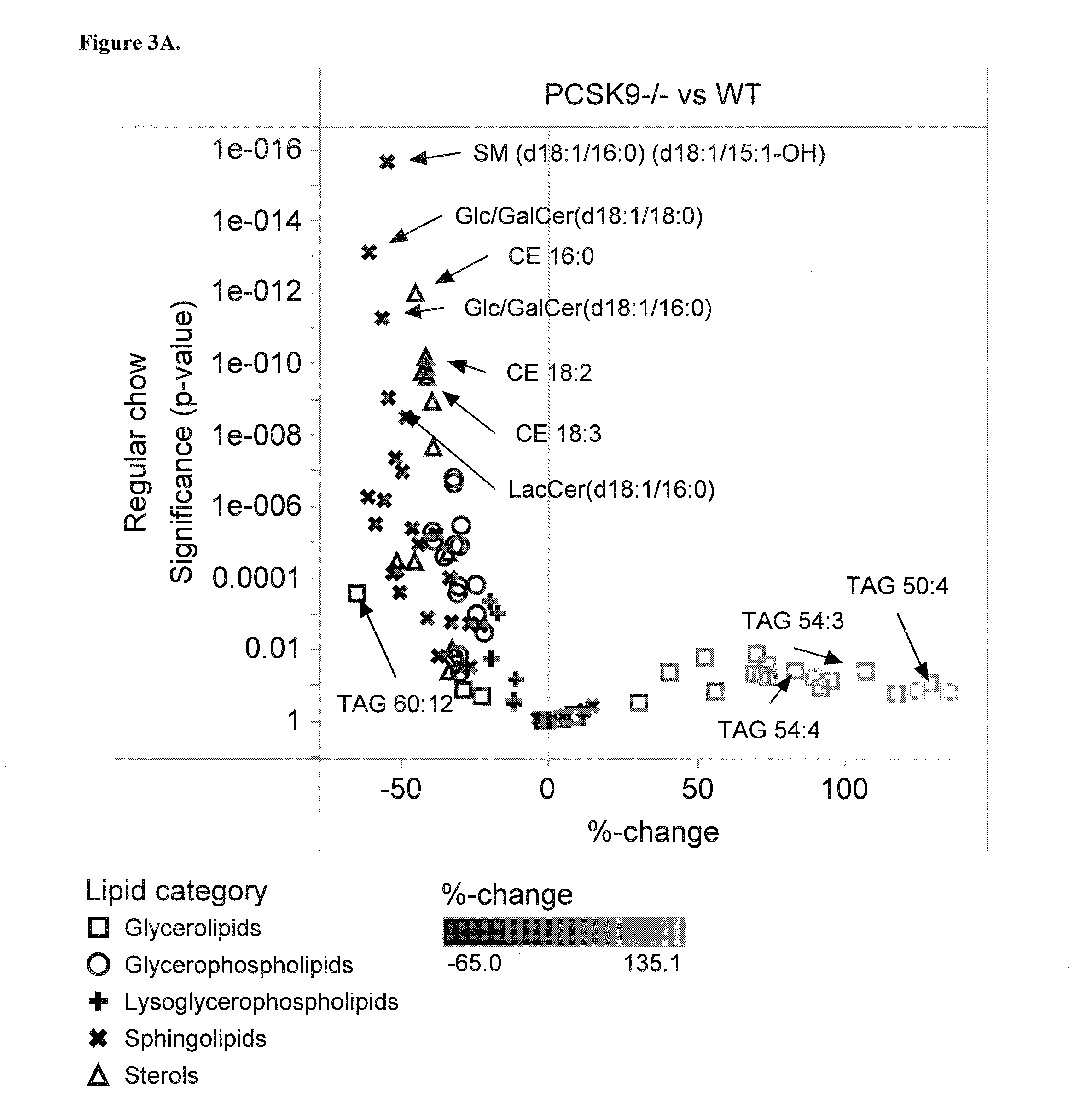
Sensitive Efficacy and Specificity Biomarkers for Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibition
InactiveUS20150160247A1Lower beneficialLower essential lipidCompound screeningApoptosis detectionKexinSubtilisin
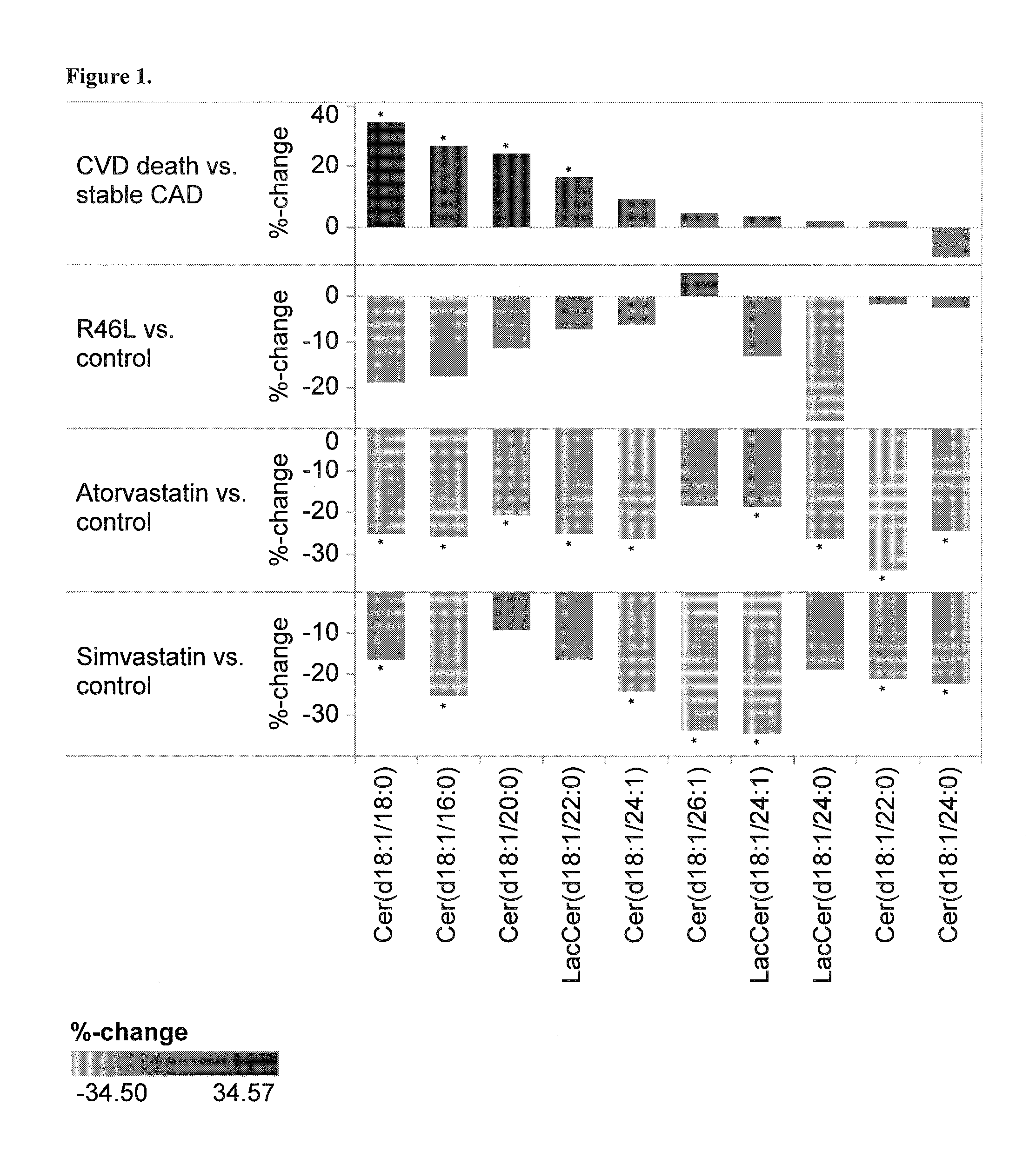
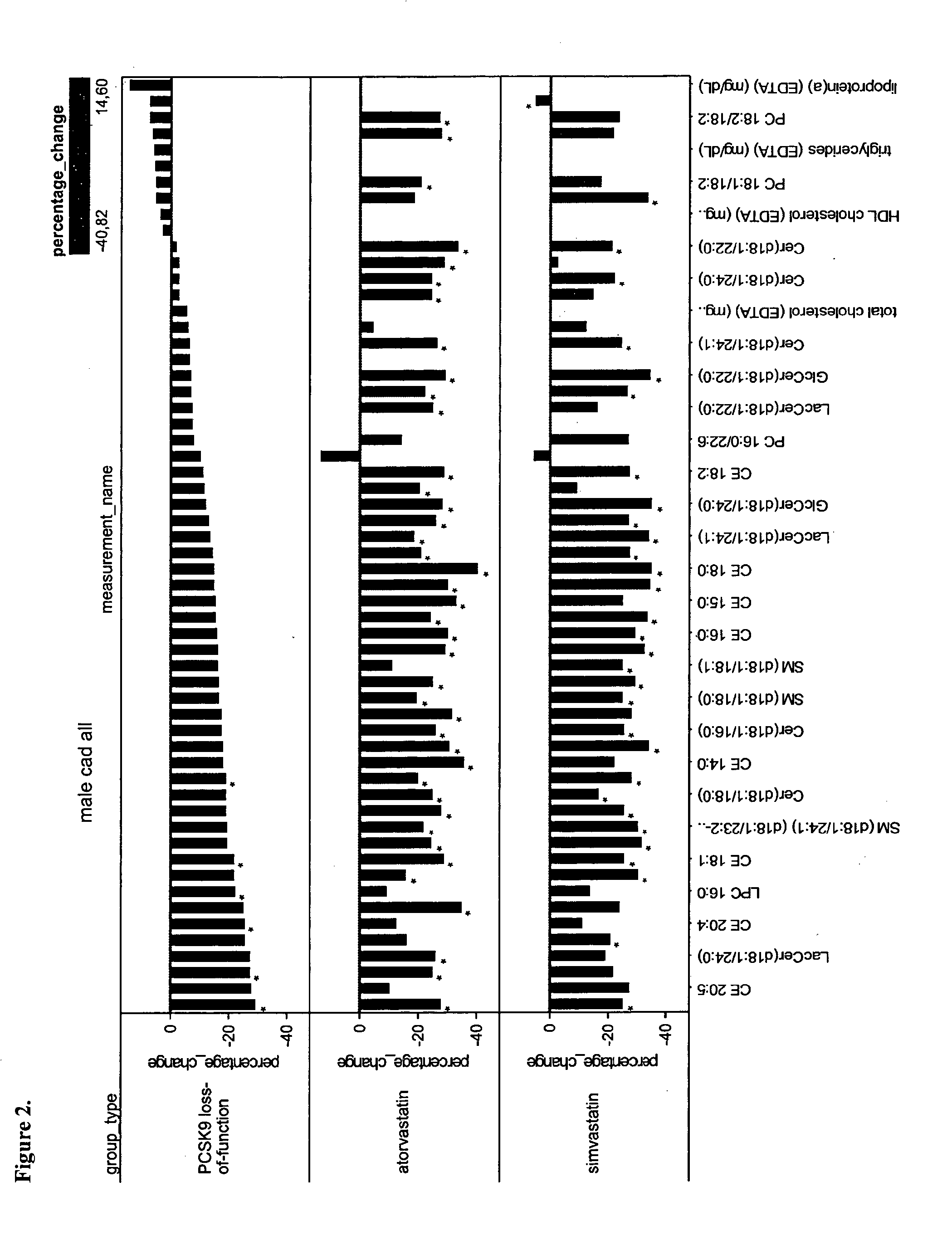
The present invention inter alia provides a method, and uses thereof, to measure drug efficacy and specificity of treatment with an inhibitor of Proprotein Convertase Subtilisin / Kexin Type 9 (PCSK9) by detecting the concentrations of lipids and / or lipid-lipid concentration ratios of a biological sample and comparing it to a control. The invention is applicable, inter alia, to determining whether a PCSK9 inhibiting drug is functioning efficiently in lowering serum low-density lipoprotein (LDL) concentration and whether a PCSK9 inhibiting drug displays any adverse side-effects, such as liver toxicity. Provided are lipid markers that are more specific and sensitive in detecting drug efficacy and possible adverse drug-induced side-effects than the currently utilized clinical markers. Also provided is an antibody towards said lipids, and the use thereof for predicting and diagnosing of PCSK9 inhibiting drug-induced adverse reactions. The invention additionally relates to kits comprising lipids and / or an antibody thereto, for the determination of PCSK9 inhibiting drug efficacy and drug-induced adverse reactions.
Owner:ZORA BIOSCIENCES OY
AX1 PCSK9 antagonists
Antagonists of human proprotein convertase subtilisin-kexin type 9 (“PCSK9”) are disclosed. The disclosed antagonists are effective in the inhibition of PCSK9 function and, accordingly, present desirable antagonists for use in the treatment of conditions associated with PCSK9 activity. The present invention also discloses nucleic acid encoding said antagonists, vectors, host cells, and compositions comprising the antagonists. Methods of making PCSK9-specific antagonists as well as methods of using the antagonists for inhibiting or antagonizing PCSK9 function are also disclosed and form important additional aspects of the present disclosure.
Owner:MERCK SHARP & DOHME LLC
A method for constructing a gene detection library for familial hypercholesterolemia and its kit
ActiveCN109517884BHigh precisionEasy to operateMicrobiological testing/measurementLibrary creationGenes mutationDimer
Owner:ANNGEEN BIOTECHNOLOGY CO LTD
Methods of reducing a viral infection and kits therefore
A method for treating and / or preventing a proprotein convertase subtilisin / kexin type 9 preproprotein (PCSK9)-susceptible viral infection comprising increasing a PCSK9 activity and / or expression in a biological system infected by the virus, whereby the increased PCSK9 activity and / or expression treats and / or prevents the viral infection in the biological system. Methods of classifying subjects, methods of screening and kits therefore.
Owner:INSTITUT NATIONAL DE LA RECHERCHE SCIENTIFIQUE +1
Dosing regimens for use with PCSK9 inhibitors
ActiveUS10428157B2Reducing LDL-CGain is not constantMetabolism disorderAntibody ingredientsKexinDisease
Owner:SANOFI BIOTECH SAS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com