Antagonists of pcsk9
An antagonist, antagonism technology, applied in the direction of antibodies, anti-enzyme immunoglobulins, metabolic diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
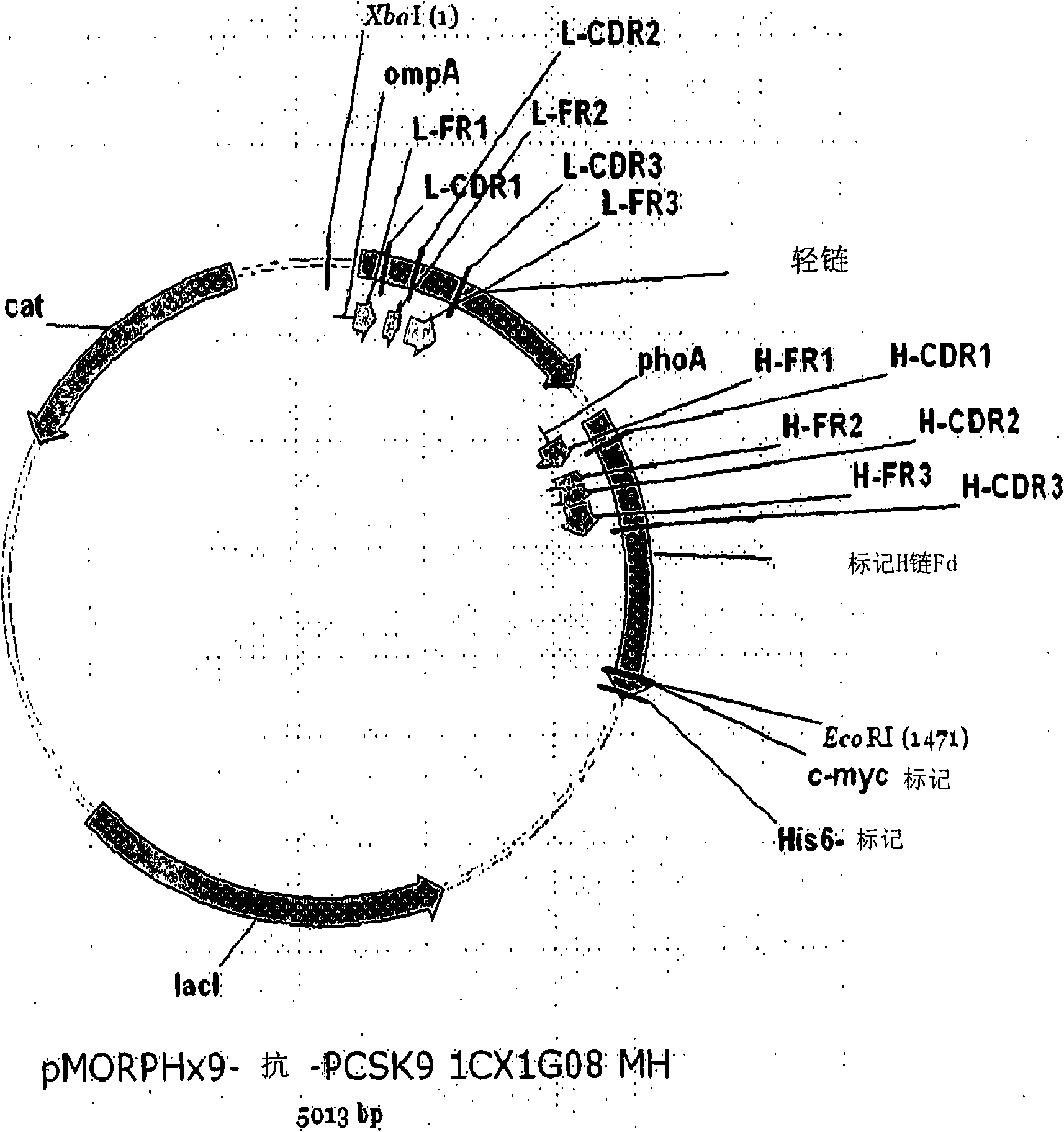
[0113] Isolation of recombinant Fab displaying phage
[0114] A recombinant Fab phage display library was panned against immobilized recombinant human PCSK9 (see, for example, Knappik et al., 2000 J. Mol. Biol. 296:57-86), and the method used was briefly described as follows: first, the phage Fab display library was divided into three pools ( pool): One pool is VH2+VH4+VH5, another pool is VH1+VH6, and the third pool is VH3. Phage libraries and immobilized PCSK9 protein were blocked with nonfat dry milk.
[0115] For the first round of panning, each phage pool was independently bound to V5-, histidine-tagged PCSK9 protein in wells immobilized on Nunc Maxisorp plates. The immobilized phage-PCSK9 complex was sequentially washed with the following reagents: (1) PBS / 0.5% Tween TM 20 (three quick washes); (2) PBS / 0.5% Tween TM 20 (one 5 minute incubation with moderate shaking); (3) PBS (three quick washes); and (4) PBS (two 5 minute incubations with moderate shaking). Bound pha...
Embodiment 2
[0120] ELISA screening of bacterially expressed FABS
[0121] Cultures of each transformant were induced with IPTG and grown overnight to express the Fab. Culture supernatants (candidate Fabs) were incubated with purified V5-, histidine-tagged PCSK9 protein immobilized in wells of a 96-well Nunc Maxisorp plate, prepared in PBS with a plate washer 0.1% of Tween20 TM Wash, incubate with HRP-conjugated anti-Fab antibody, again with PBS / Tween20 TM washing. Bound HRP was detected by adding TMP substrate, and the A of each well was read with a plate reader. 450 value.
[0122] Include the following negative controls:
[0123] A control for non-specific Fab binding on each plate was incubated with a parallel expressed anti-EsB (an unrelated Fab) preparation.
[0124] growth medium only.
[0125] Include positive controls for ELISA and Fab expression as follows;
[0126] EsB antigen was allowed to bind to three wells of the plate and then incubated with anti-EsB Fab. For a co...
Embodiment 3
[0128] DNA Sequencing of PCSK9 ELISA-Positive FAB Clones
[0129] Bacterial cultures for DNA preparation were obtained by inoculating 1.2 ml of 2xYT broth containing chloramphenicol in a master glycerol stock of positive Fabs and growing overnight. DNA was prepared from cell pellets obtained by centrifugation from overnight cultures using a Qiagen Turbo Mini prep procedure performed on a BioRobot 9600. ABI dye terminator (DyeTerminator) cycle sequencing was performed on the DNA on the ABI 3100 Genetic Analyzer (Genetic Analyzer) with the sequencing primers determined by Morphosys to obtain the DNA sequence of the Fab clone. The DNA sequences were compared to each other to determine unique clone sequences and to determine the light and heavy chain subtypes of the Fab clones.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com