PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) inhibitor in preparing medicine for treating T-cell medicated inflammatory-immune diseases
An inflammatory immune and inhibitory technology, applied in the field of medicine and biology, can solve the problems that the metabolic disorder of psoriasis patients cannot be effectively improved, and the therapeutic targets for psoriatic skin lesions and associated metabolic abnormalities cannot be found, and the therapeutic effect is remarkable. , Small side effects, small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
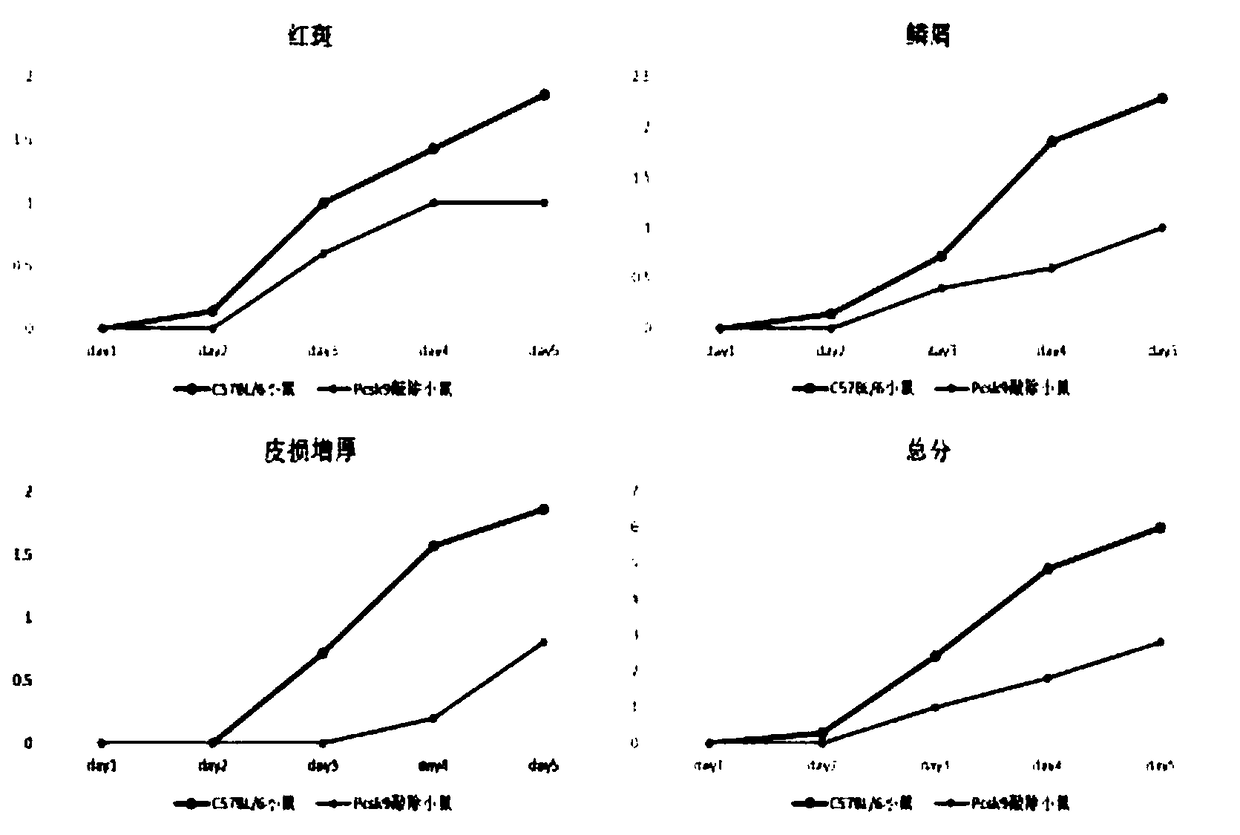
[0046] PCSK9 knockout significantly alleviates IMQ-induced psoriasis-like inflammatory response in mice
[0047] Main reagents: 5% imiquimod cream (IMQ), PCSK9 primary antibody (abcam company), NF-kB primary antibody (abcam company)
[0048] Experimental animals: C57BL / 6 (B6) mice, 7 males; C57BL / 6-PCSK9- / - mice, 5 males and 5 males. These mice were purchased from The Jackson Laboratory, Maine, USA.
[0049] experimental method:
[0050] (1) After hair removal on the back of two groups of mice with different genotypes, 62.5 mg of 5% imiquimod cream was applied every day for 5 consecutive days.
[0051] (2) Make scores (erythema, scales, thickening of skin lesions and total score) every day before and after application of the drug, and take photos for archiving (the scores are averaged by two researchers).
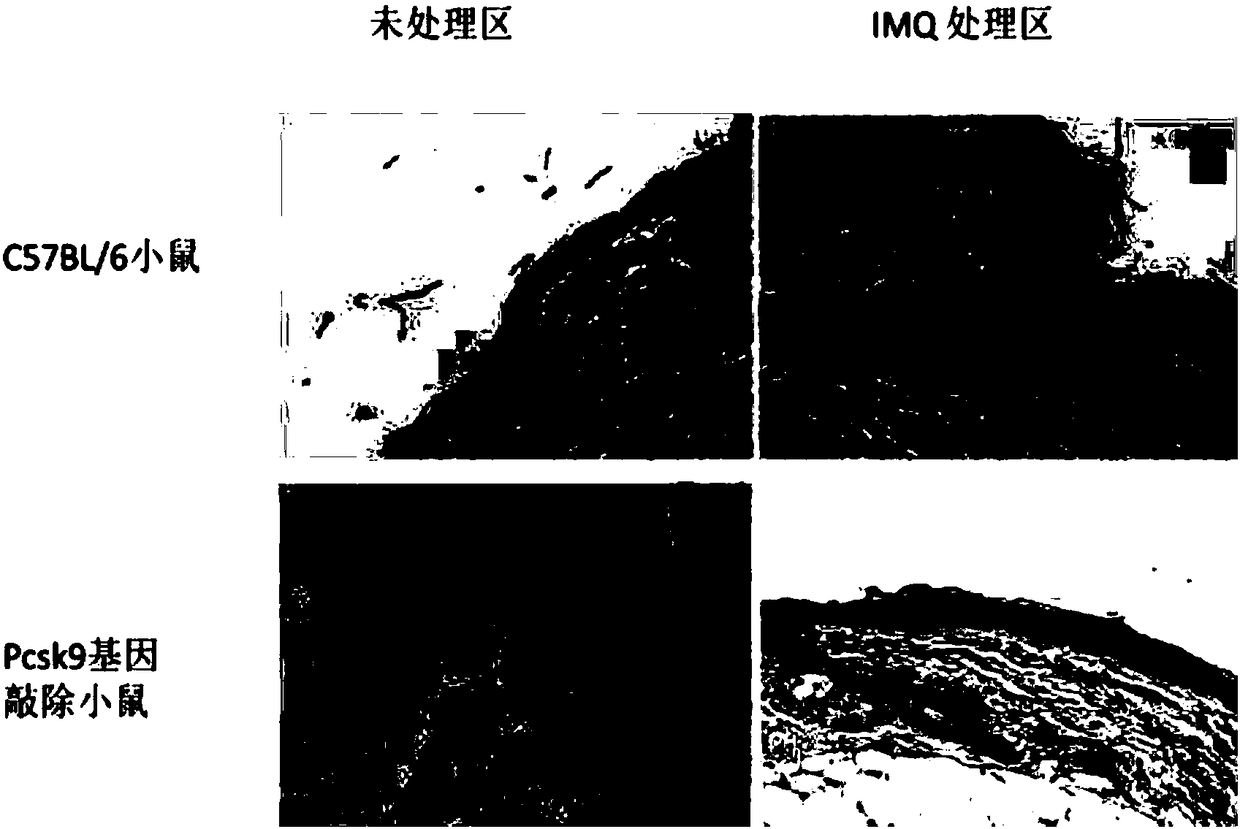
[0052] (3) On the last day of the experiment, all mice were sacrificed, and back skin tissues (treated area and non-treated area) were collected.
[0053] (4) HE staini...
Embodiment 2
[0060] si-PCSK9 transfection enhances apoptosis and inhibits proliferation of human keratinocytes
[0061] Experimental materials and reagents:
[0062] (1) Human primary keratinocytes (Lifeline Cell Technology, FC-0064);
[0063] (2) DermaLife keratinocyte culture medium (Lifeline Cell Technology, LL-0007), si-PCSK9 (Santa Cruz, sc-45482), Lipofectamine 3000 transfection reagent (ThermalFisher, L-3000001), AnnexinV (BD), PI (BD).
[0064] experimental method:
[0065] (1) Cultivate primary human keratinocytes and plant them in 6-well plates. When the cell density is 60-70%, perform si-RNA (si-Con&si-PCSK9) transfection. After transfection, 24h / 48h / 72h respectively The cell viability of each well was measured by the MTT method, three wells were counted at each time point of the two groups of cells, the average value was taken, and the cell viability curve was drawn;
[0066] (2) Cultivate primary human keratinocytes and plant them in 6-well plates. When the cell density is...
Embodiment 3
[0071] The expression of PCSK9 in skin lesions of patients with psoriasis was significantly higher than that of non-lesional and normal controls
[0072] Experimental materials: skin lesions and non-lesions of 30 cases of psoriasis patients and 30 cases of normal human skin, PCSK9 antibody (abcam company).
[0073] Experimental methods: immunohistochemistry, real-time quantitative-PCR.
[0074] Experimental results: The lesions and non-lesions of the patients were obtained by drilling, and the normal skin was obtained from the excess skin of cosmetic surgery. The results of immunohistochemistry showed that the expression of PCSK9 in the skin lesions of psoriasis patients was significantly higher than that of non-skin lesions and normal controls; PCSK9-positive cells were mainly distributed in the epidermis and the cells near the epidermis in the dermis, and in the cells in the dermal blood vessels. no expression (see Figure 8 ). RNA was extracted after skin homogenization,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com