Methods for Producing Polypeptidies in Protease-Deficient Mutants of Trichoderma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
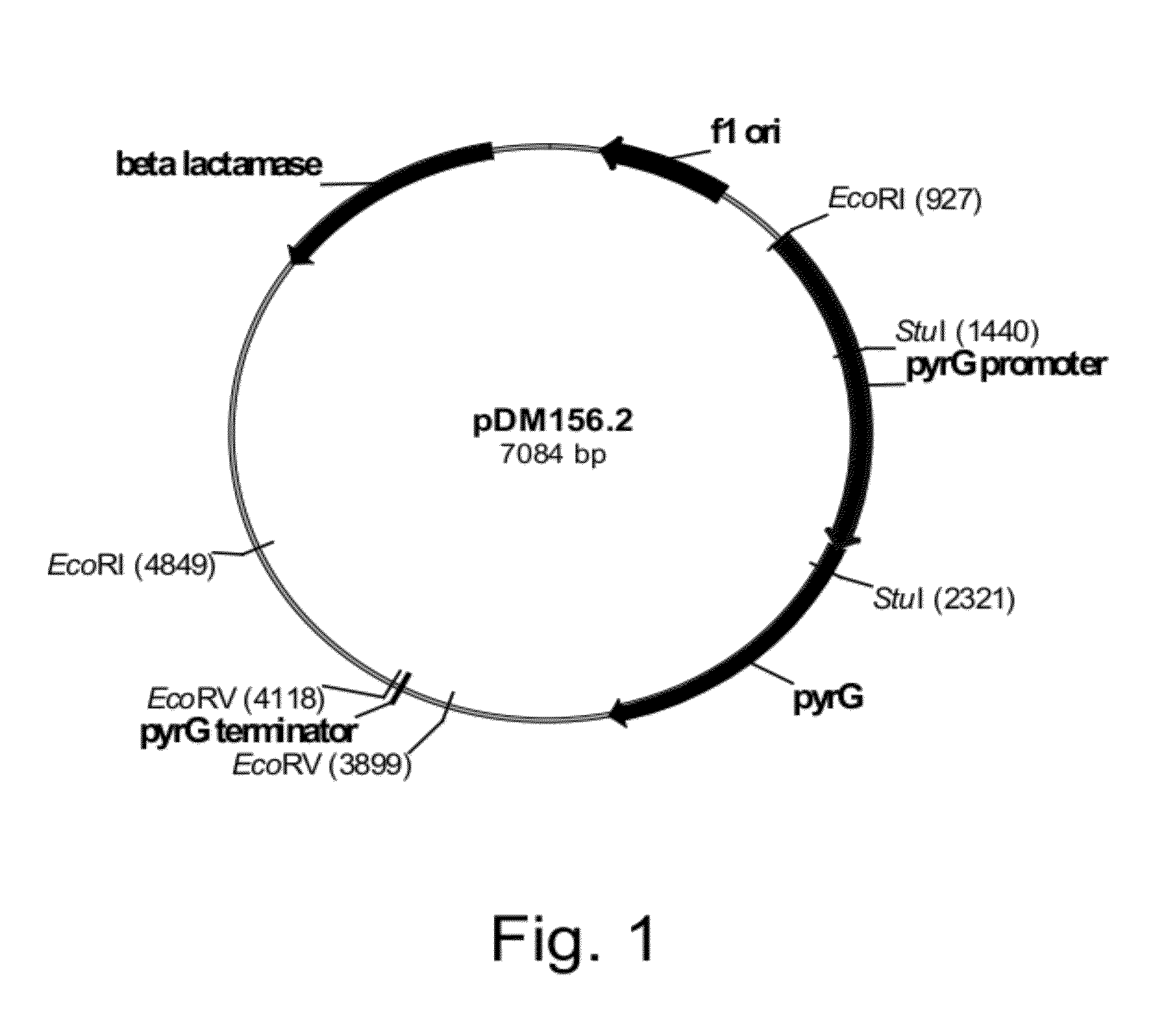
Construction of Plasmid pDM156.2
[0174]A probe of a Neurospora crassa orotidine-5′-monophosphate decarboxylase (pyr-4) gene (SEQ ID NO: 7 for the DNA sequence and SEQ ID NO: 8 for the deduced amino acid sequence) was prepared by PCR incorporating digoxigenin-labeled deoxyuridine-triphosphate (dUTP) using the primers described below.
Primer (sense):(SEQ ID NO: 9)5′-GTCAGGAAACGCAGCCACAC-3′Primer (anti-sense):(SEQ ID NO: 10)5′-AGGCAGCCCTTGGACGACAT-3′
[0175]Plasmid pFB6 (Buxton et al, 1983, Molecular and General Genetics 190: 403-405) was digested with Hind III and the digestion purified by 1% agarose gel electrophoresis using 40 mM Tris base-20 mM sodium acetate-1 mM disodium EDTA (TAE) buffer. A 1.1 kb pyr-4 fragment was excised from the gel and extracted using a QIAQUICK® Gel Extraction Kit (QIAGEN Inc., Valencia Calif., USA) according to the manufacturer's suggested protocols.
[0176]The amplification reaction (50 μl) was composed of 1×Taq DNA Polymerase Buffer (New England Biolabs Inc.,...
example 2
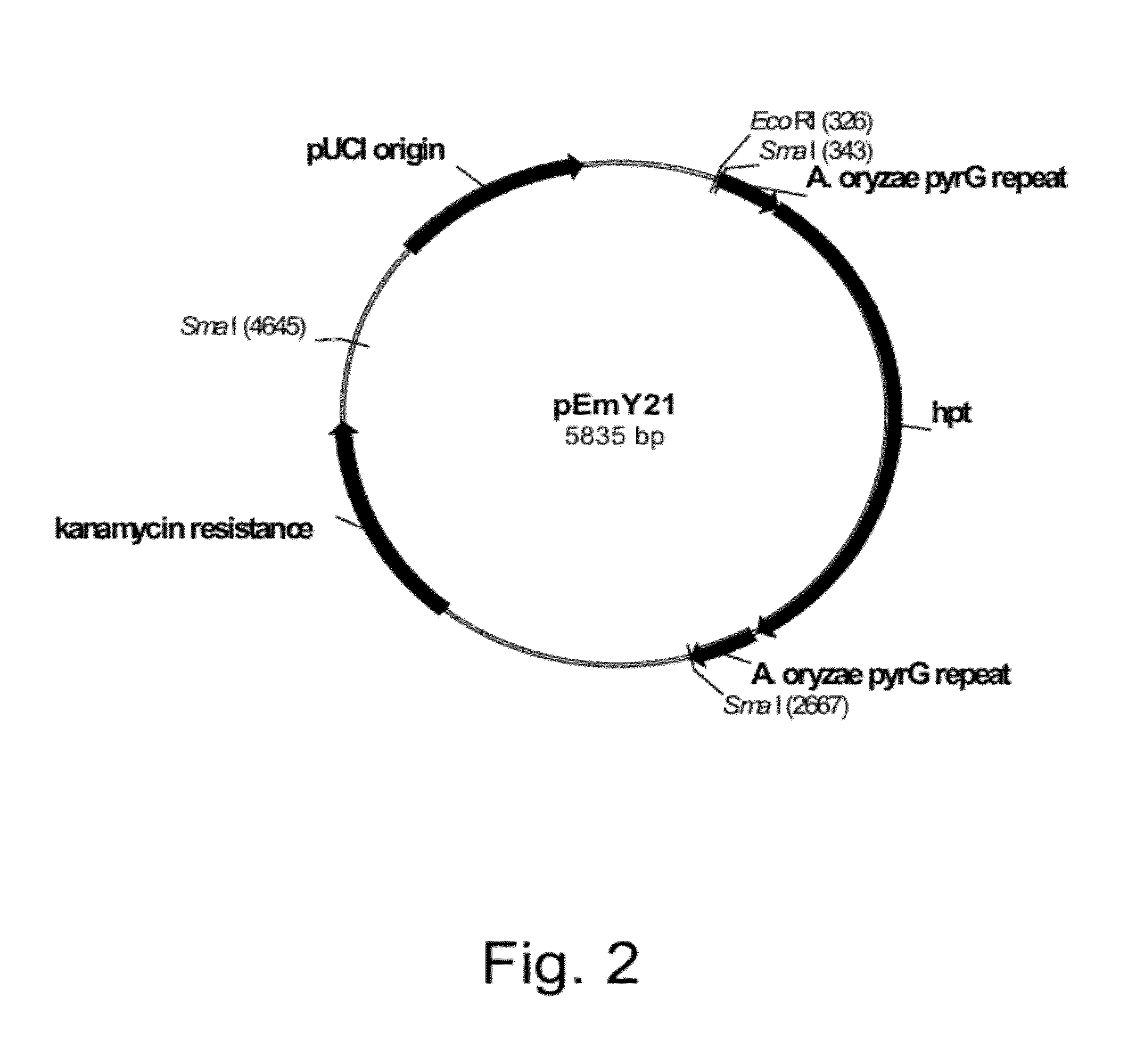
Construction of Plasmid pEmY21
[0182]An E. coli hygromycin phosphotransferase (hpt) gene (SEQ ID NO: 11 for the DNA sequence and SEQ ID NO: 12 for the deduced amino acid sequence) was amplified from plasmid pPHTI (Cummings et al., 1999, Current Genetics 36: 371-382) using the following primers:
Forward primer:(SEQ ID NO: 13)5′-GGGttcgaaTTCATTTAAACGGCT-3′Reverse primer:(SEQ ID NO: 14)5′-GGGagcgctCAATATTCATCTCTC-3′
The restriction enzyme sites Bst BI (forward primer) and Eco 47111 (reverse primer) were engineered into the primers, represented by the underlined sequence, for cloning.
[0183]The PCR reaction (to amplify the hpt gene) was composed of 1×ThermoPol reaction buffer (New England Biolabs, Inc, Ipswich, Mass., USA), 200 μM dNTPs, 50 pmol of the forward and reverse primers, 100 pg of pPHT1, 1 unit of VENT® DNA polymerase (New England Biolabs Inc., Ipswich, Mass. USA), and sterile distilled water in a total volume of 100 μl. The amplification reaction was performed using a ROBOCYCLER®...
example 3
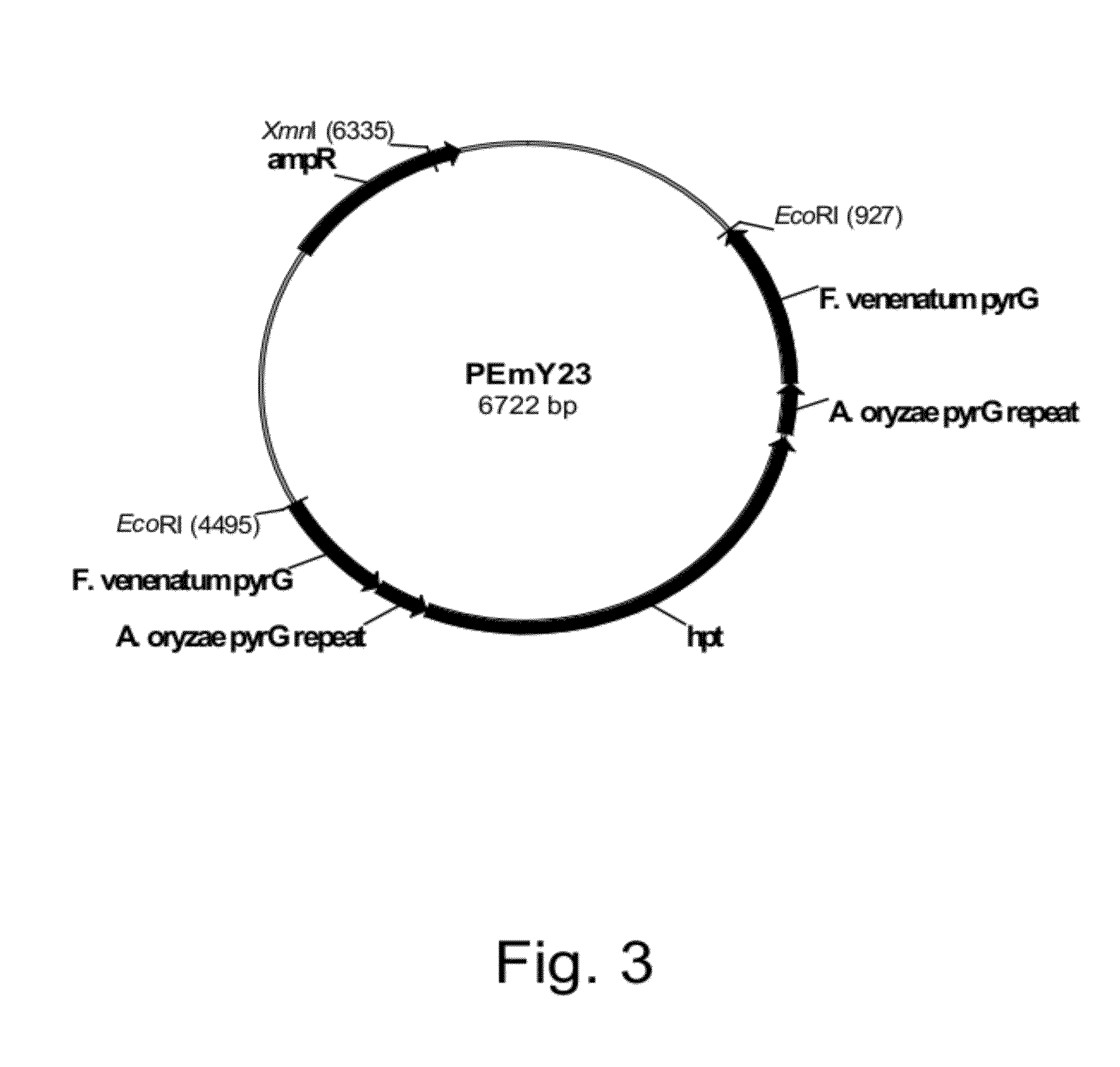
Construction of Plasmid pEmY23
[0191]The Fusarium venenatum pyrG coding sequence (2,678 bp) was excised from pDM156.2 (Example 1) by digestion with Eco RV and Stu I restriction endonucleases, and the remaining 4,398 bp vector was gel-purified using a QIAQUICK® Gel Extraction Kit according to the manufacturer's directions. The Sma I fragment of pEmY21 was isolated and gel-purified using a QIAQUICK® Gel Extraction Kit and the two gel-purified fragments were ligated together. They were screened for insert orientation, sequenced for the absence of errors, and one of the clones with the correct insert sequence was selected and designated pEmY23 (FIG. 3).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com