Methods for Treating Patients with Hyperlipidemia by Administering a PCSK9 Inhibitor in Combination with an ANGPTL3 Inhibitor
a technology of angptl3 inhibitor and hyperlipidemia, which is applied in the direction of drug composition, antibody medical ingredients, metabolic disorders, etc., can solve the problems of many high-risk patients not reaching the ldl-c level as the guideline target, and achieve the effect of reducing the serum lipoprotein level and reducing the risk of atherosclerosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of Human Antibodies to Human PCSK9
[0130]Human anti-PCSK9 antibodies were generated as described in U.S. Pat. No. 8,062,640. The exemplary PCSK9 inhibitor used in the following Example is the human anti-PCSK9 antibody designated “H1H316P,” also known as “alirocumab”, or “PRAULENT®”. H1 H316P has the following amino acid sequence characteristics: a heavy chain comprising SEQ ID NO:16 and a light chain comprising SEQ ID NO:20; a heavy chain variable region (HCVR) comprising SEQ ID NO:12 and a light chain variable domain (LCVR) comprising SEQ ID NO:17; a heavy chain complementarity determining region 1 (HCDR1) comprising SEQ ID NO:13, a HCDR2 comprising SEQ ID NO:14, a HCDR3 comprising SEQ ID NO:15, a light chain complementarity determining region 1 (LCDR1) comprising SEQ ID NO:18, a LCDR2 comprising SEQ ID NO:19 and a LCDR3 comprising SEQ ID NO:21.
example 2
Generation of Human Antibodies to Human ANGPTL3
[0131]Human anti-ANGPTL3 antibodies were generated as described in U.S. Pat. No. 9,018,356. The exemplary ANGPTL3 inhibitor used in the following Example is the human anti-ANGPTL3 antibody designated “H4H1276S,” also known as “evinacumab.” H4H1276S has the following amino acid sequence characteristics: a heavy chain comprising SEQ ID NO:10 and a light chain comprising SEQ ID NO:11; a heavy chain variable region (HCVR) comprising SEQ ID NO:2 and a light chain variable domain (LCVR) comprising SEQ ID NO:3; a heavy chain complementarity determining region 1 (HCDR1) comprising SEQ ID NO:4, a HCDR2 comprising SEQ ID NO:5, a HCDR3 comprising SEQ ID NO:6, a light chain complementarity determining region 1 (LCDR1) comprising SEQ ID NO:7, a LCDR2 comprising SEQ ID NO:8 and a LCDR3 comprising SEQ ID NO:9.
example 3
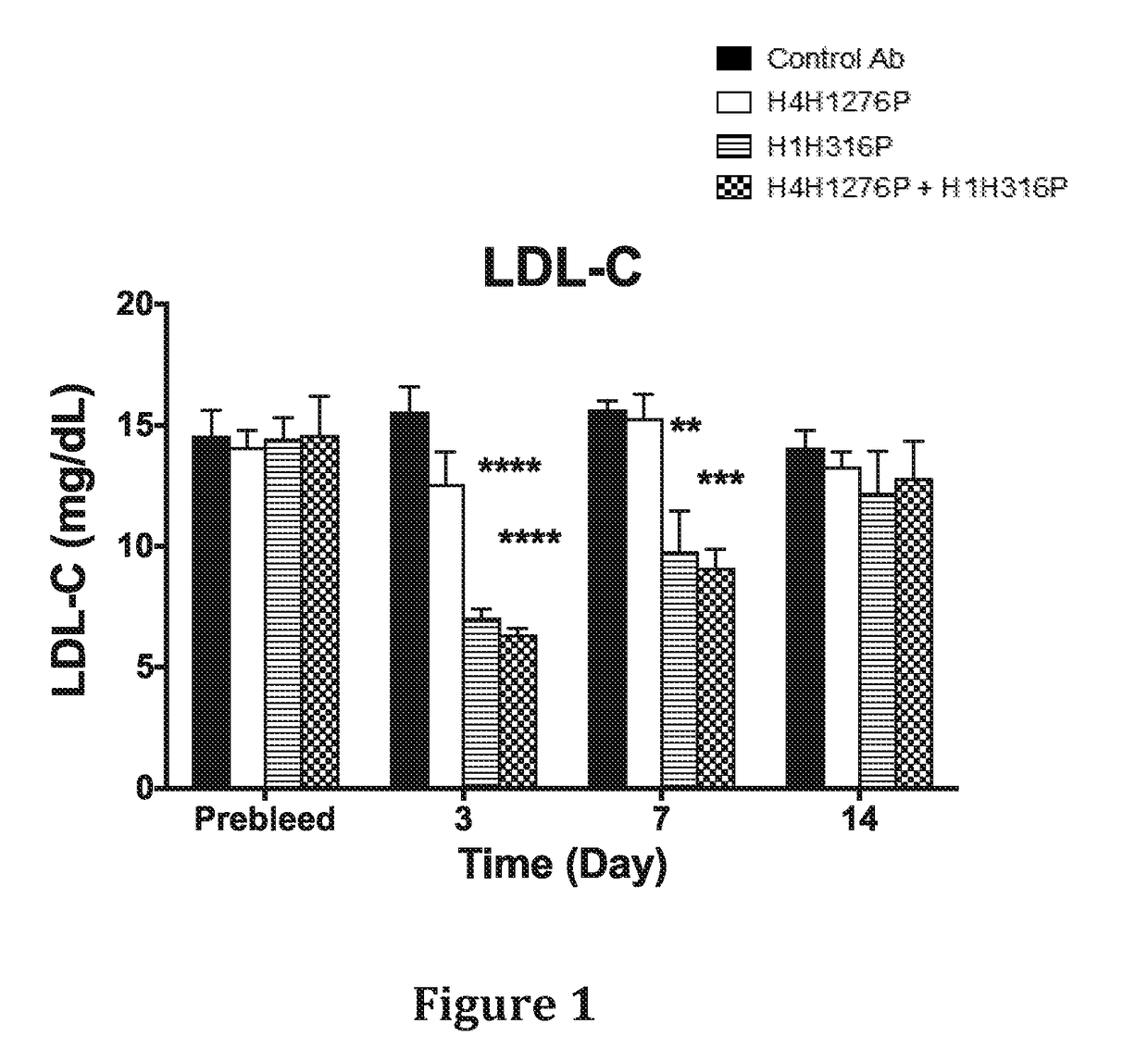
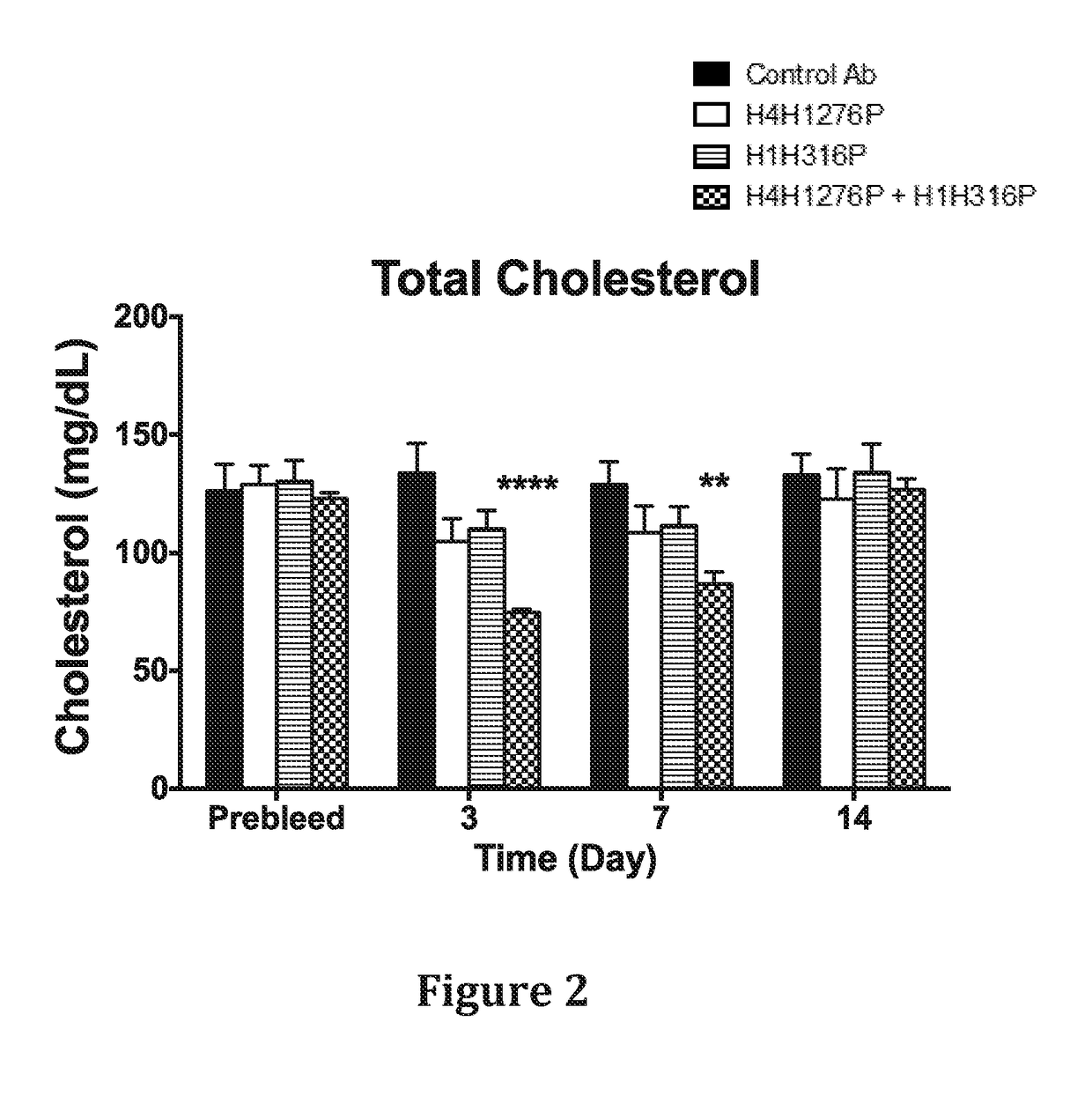
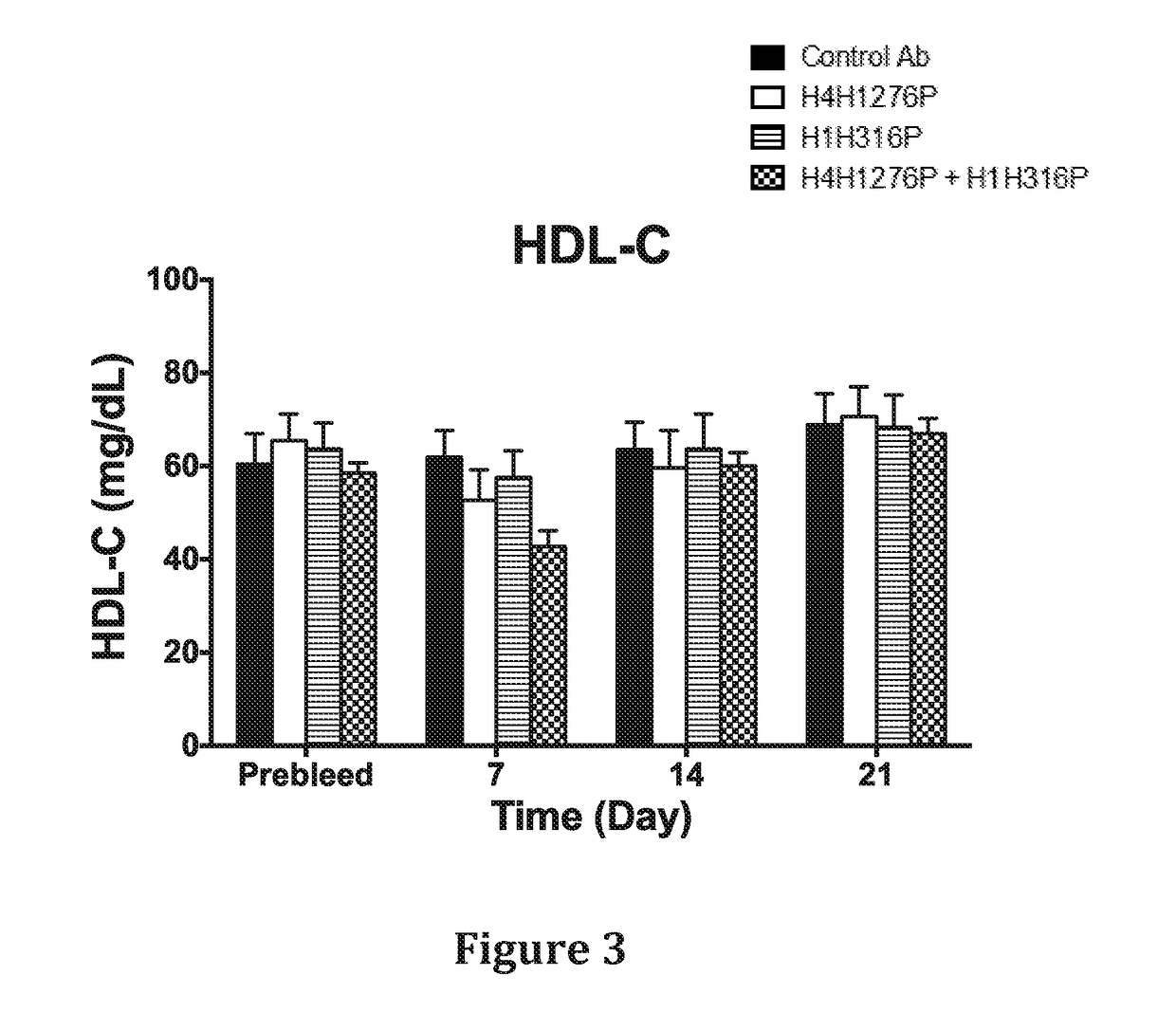
In Vivo Effect of Treatment with a Combination of an Anti-hANGPTL3 Antibody and an Anti-PCSK9 Antibody on Circulating Lipid Levels in Hyperlipidemic Ldlr− / + mice
[0132]The effect of anti-hANGPTL3 antibody H4H1276 (evinacumab) alone, anti-PCSK9 antibody H1H316P (alirocumab) alone and both antibodies in combination on serum lipids levels was determined in LDLR - / ±mice. These mice are hyperlipidemic with majority of their circulating cholesterol found in the form of LDL due to partial deficiency in LDLR, the major receptor for LDL-C uptake.
[0133]In the first study, male LDLR− / + mice on chow diet were pre-bled 5 days before the experiment and mice were put into groups of five mice each. The antibodies, H4H1276P, H1H316P, their combination and isotype-matched (hlgG4) control with irrelevant specificity, were administered at a dose of 10mg / kg each by subcutaneous injection on Day 0 of the study. Mice were bled after 4 hours of fasting on consecutive days after the antibodies injections and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com