Acylhydrazone schiff base-copper complex-human serum albumin complex and applications thereof
A technology of acylhydrazone Schiff base copper and human serum albumin, applied in the field of pharmaceuticals, can solve the problems of premature release of metal prodrugs, inability to reach cancer cells, side effects, etc., to achieve not easy to be swallowed, good anticancer activity, Effect of Narrow Particle Size Distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
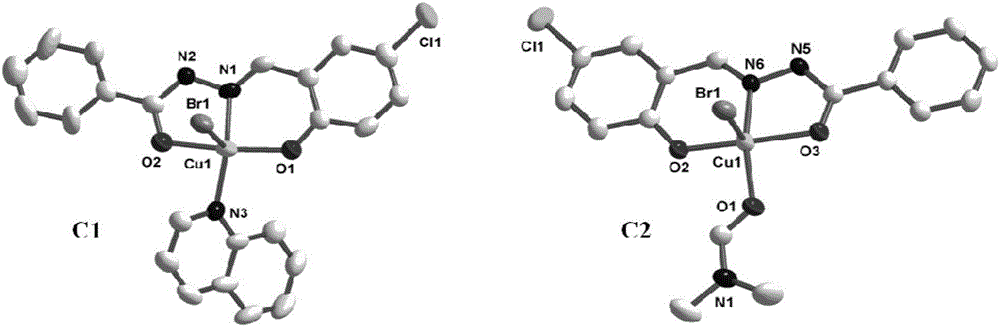
[0048] Example 1 Preparation and characterization of the acylhydrazone Schiff base copper complex [CuBr(BA)(L)] (compound C1) and [CuBr(DMF)(L)] (compound C2) of the present invention
[0049] The acylhydrazone Schiff base ligand L used in the present invention is based on (E)-N'-(5-chloro-2-hydroxybenzylidene) benzoylhydrazide Schiff base (references: Ali HM, Puvaneswary S, Ng SW.5-Chlorosalicylaldehydebenzoylhydrazone.Acta Crystallogr., Sect.E: Struct.Rep.Online 2005,61,o2415.) as an example to prepare the acylhydrazone Schiff base copper complex.
[0050] [CuBr(BA)(L)](C1) was synthesized as follows: L (0.55 g, 2 mmol) and the heterocyclic nitrogen ligand quinoline (BQAL; 0.26 g, 2 mmol) were dissolved in 15 mL of methanol and stirred at room temperature for 1 h , an orange-yellow liquid was obtained. Then add CuBr dissolved in methanol 2 (0.44g, 2mmol) solution. The above mixed solution was stirred at room temperature for another 1 h to obtain a dark green solution, and...
Embodiment 2
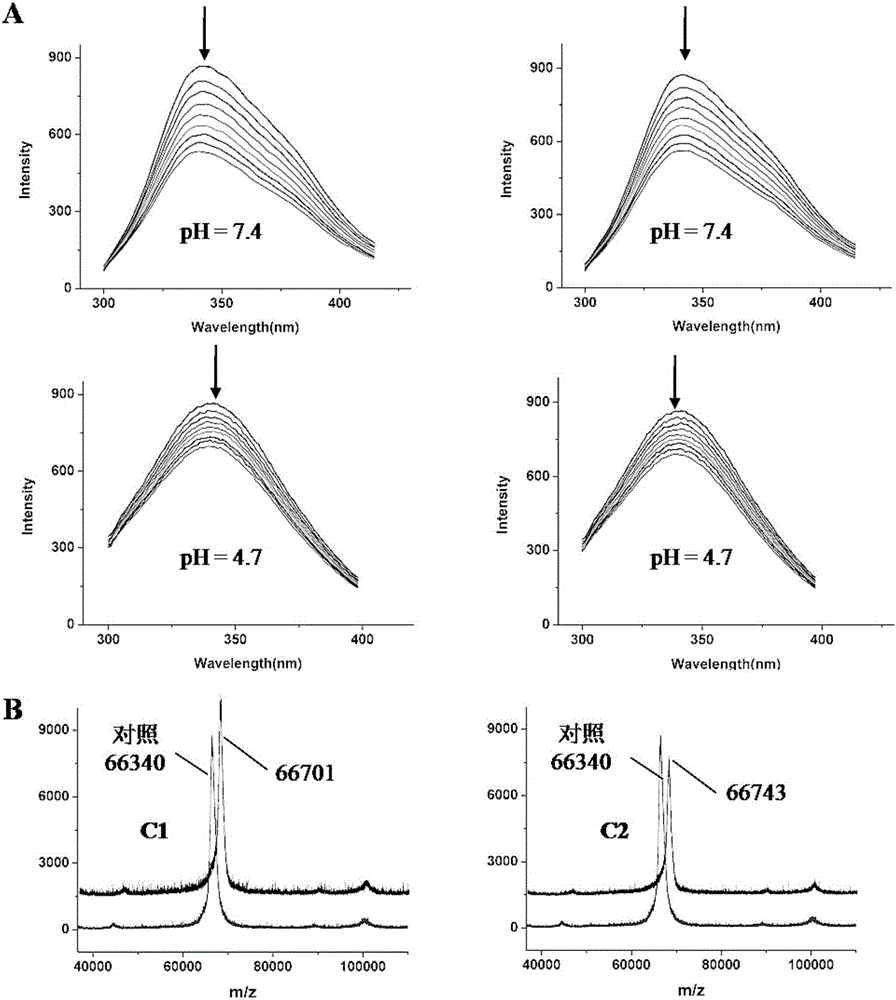
[0063] Example 2 Preparation and Characterization of Acylhydrazone Schiff Base Copper Complex-Human Serum Albumin Complex (HSA-C1 / C2)
[0064] Take 10ml of 20% medical human serum albumin, 2g powdered activated carbon and 5ml ultrapure water in a 50ml beaker, place the beaker in ice water, adjust the pH to 2.8, stir for 2h, then adjust the pH to 7.5. Then centrifuge and dialyze to remove activated carbon, dimers and polymers in HSA to obtain monomeric HSA used in subsequent experiments.
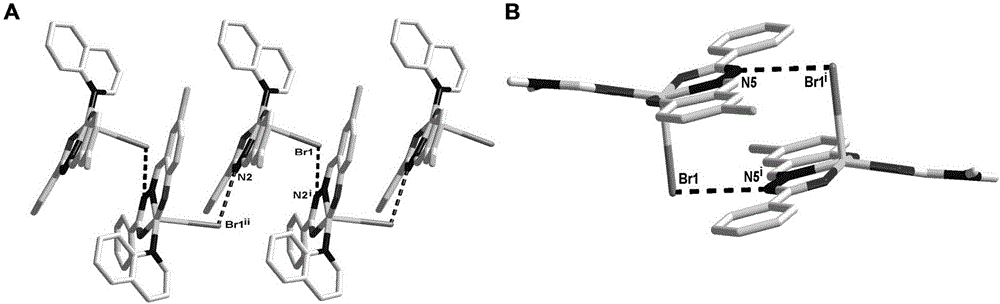
[0065] Single crystal diffraction of HSA complexes: Fatty acid (PA) was diluted to 2.5 mM with 20 mM potassium phosphate (pH 7.5). In the experiment, in order to combine C1 and C2 with HSA to form a prodrug complex, first mix 380 μL of PA (2.5 mmol), 100 μL of HSA (100 mg / mL), and 90 μL of Cu complex (5 mmol), and then Incubate for 24 hours, and finally put the mixture in a concentrator tube, wash with water, filter and concentrate to 100 mg / ml (microporous spin filter (10,000 Dalton cut-off...
Embodiment 3
[0074] Example 3 Preparation and Characterization of Acylhydrazone Schiff Base Copper Complex-Human Serum Albumin Complex-Folic Acid Nanoparticles (FA-HSA-C1 / C2)
[0075] (1) Preparation of Acylhydrazone Schiff Base Copper Complex-Human Serum Albumin Complex-Folic Acid Nanoparticles (HSA-C1 / C2 NPs)
[0076] In order to obtain the HSA-metal prodrug and a constant drug loading ratio, first the acylhydrazone Schiff base copper complex of the present invention prepared in Example 1 was incubated with HSA at room temperature (molar ratio 1:3) for 24h, and the excess The acylhydrazone Schiff base copper complex is removed by centrifugal dialysis. Next, according to the desolvation method in the literature, at room temperature and under constant stirring, adjust the pH of 4 mL of the above-incubated acylhydrazone Schiff base copper complex and HSA mixture (1.5 mM) to 8, and continuously add 15.0 mL of ethanol solution (1mL / min), gradually forming HSA-C1 / C2 NPs. After protein denatu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com