Compositions and methods for tumor-targeted delivery of effector molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
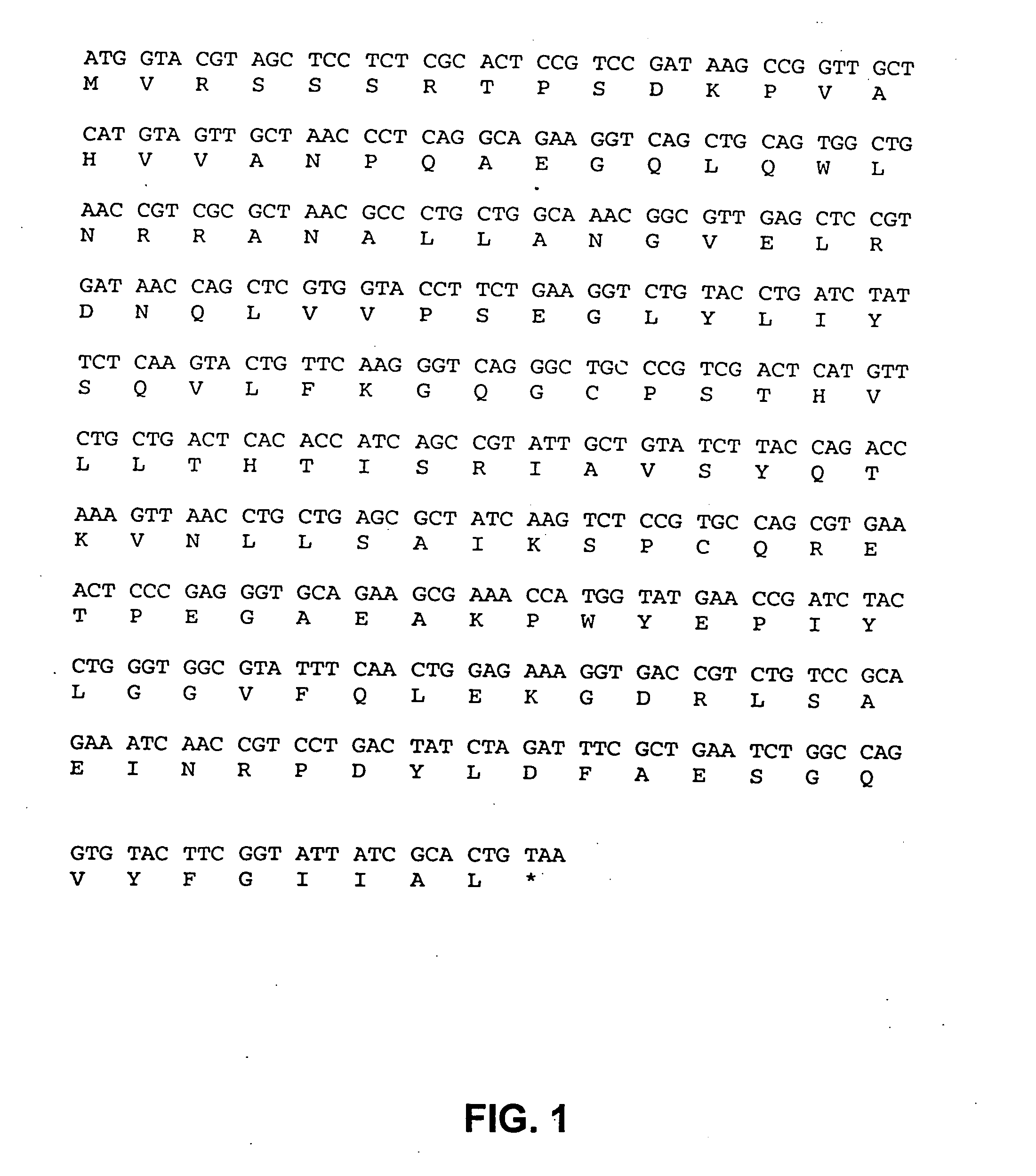
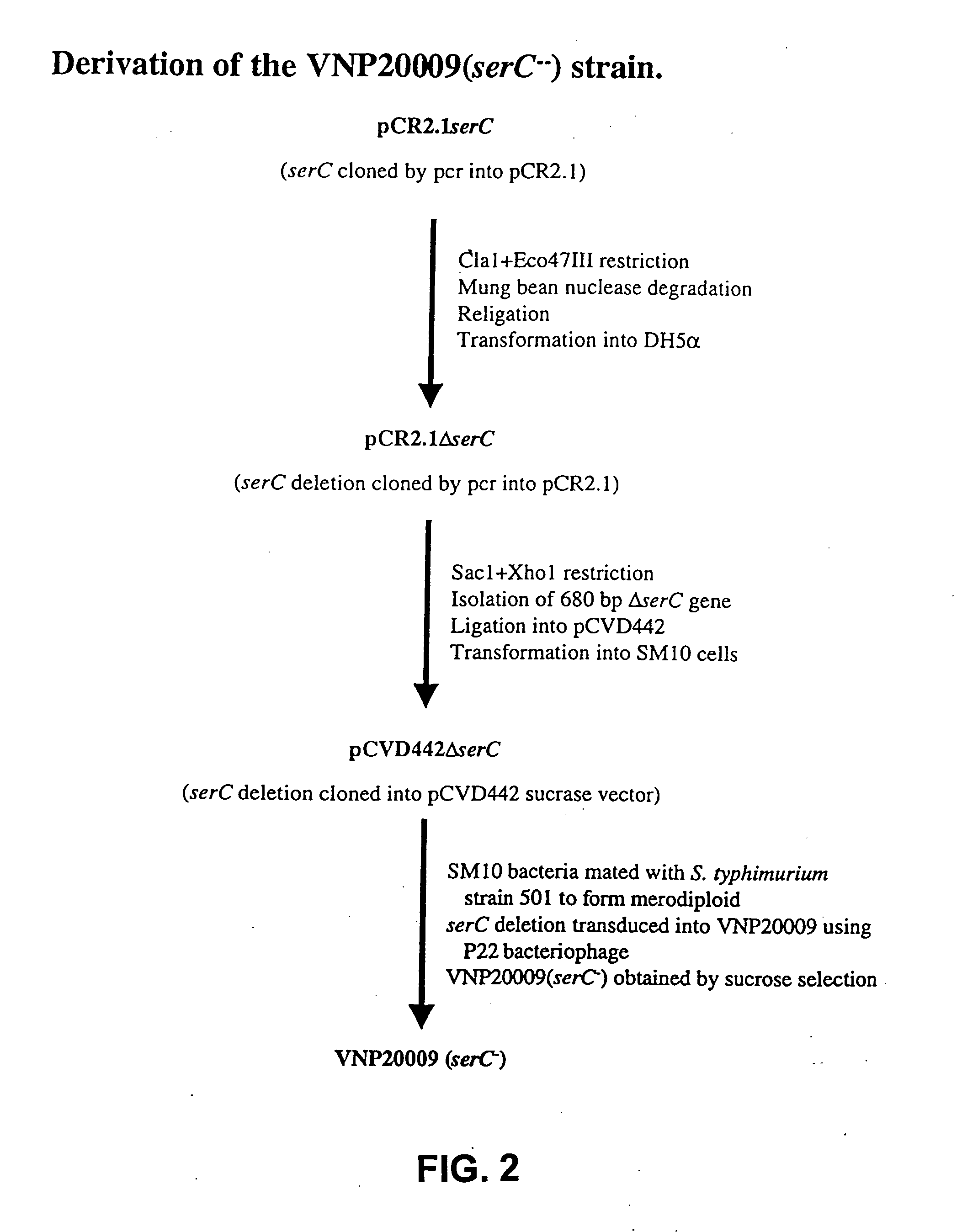
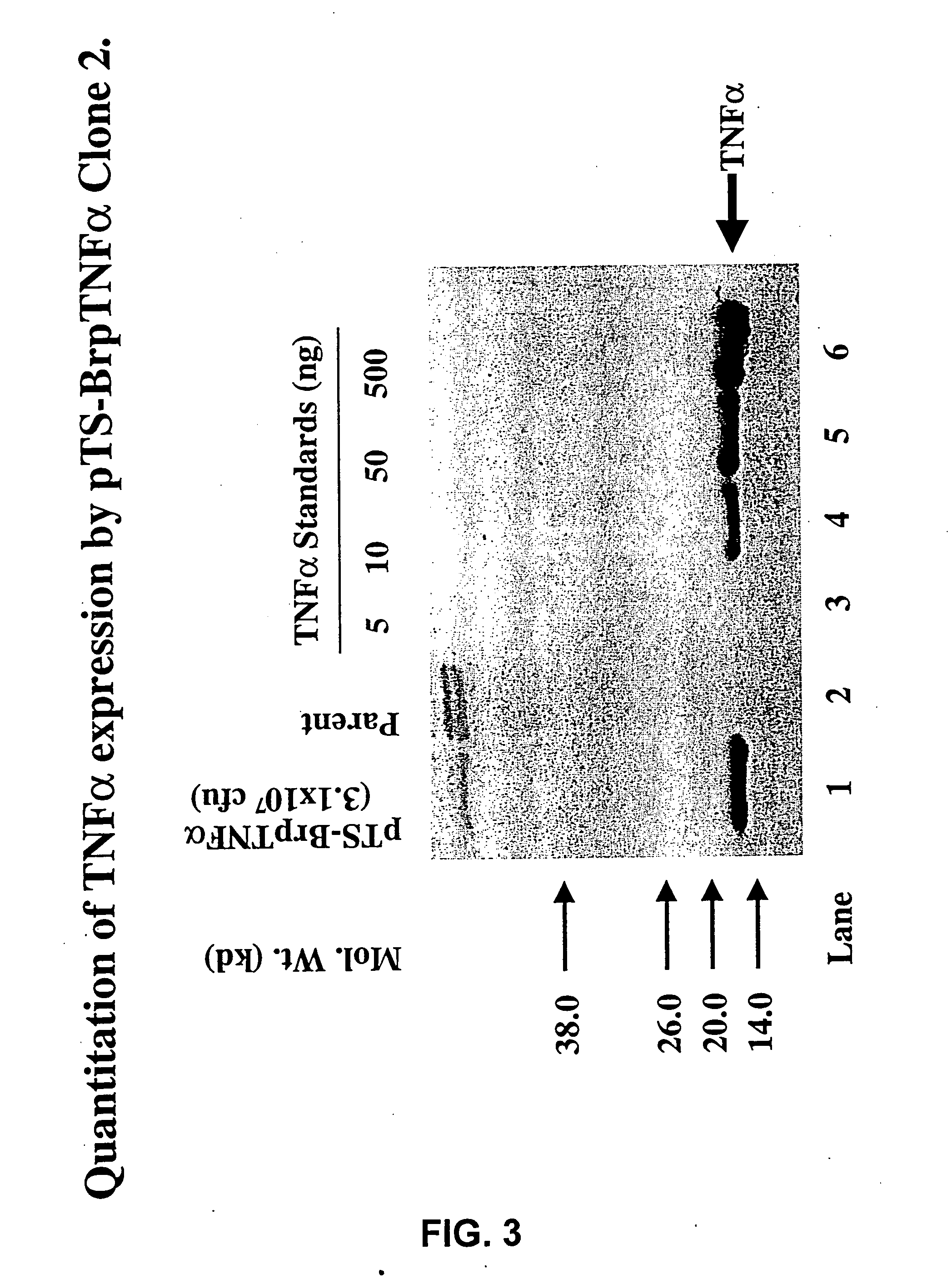
[0137] The present invention utilizes attenuated tumor-targeted strains of bacteria to deliver high levels of therapeutic primary effector molecule(s) to tumors. The present invention provides the advantage of bypassing potential systemic toxicity of certain primary effector molecules (e.g., septic shock caused by TNF-.alpha.). The present invention provides delivery of one or more primary effector molecule(s) and optionally, one or more secondary effector molecule(s) to a solid tumor. More particularly, the invention encompasses the preparation and the use of attenuated tumor-targeted bacteria, such as, e.g., Salmonella, as a vector for the delivery of one or more primary effector molecule(s) and optionally, one or more secondary effector molecule(s), to an appropriate site of action, e.g., the site of a solid tumor. Specifically, the attenuated tumor-targeted bacteria of the invention are facultative aerobes or facultative anaerobes, which are engineered to encode one or more prim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com