Continuous flow preparation method of high drug-loading microspheres
A technology of high drug loading and microspheres, which is applied in the preparation of microspheres, pharmaceutical formulations, microcapsule preparations, etc. It can solve the problems of wide particle size distribution range, average particle size, encapsulation rate drug loading, and large differences in drug release. problems, to achieve the effects of avoiding property differences, reducing toxicity risks, and reducing production costs and risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
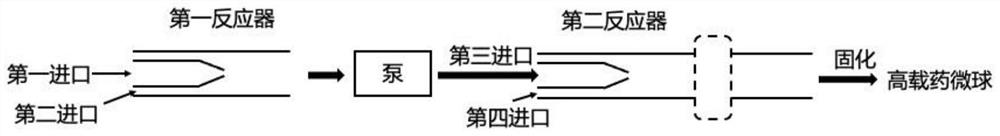
[0048] Such as figure 1 As shown, this embodiment discloses a continuous flow preparation device for highly drug-loaded microspheres, including a first reactor and a second reactor, the first reactor includes a first inlet, a second inlet and a first outlet, and the second reactor includes a first inlet, a second inlet and a first outlet. The second reactor includes a third inlet and a fourth inlet.
[0049] Optional situation 1: the first reactant and the second reactant enter the first reactor through the first inlet and the second inlet respectively, and the obtained mixture of the first reactant and the second reactant passes through the second reactor under the action of the pump. The third inlet enters the second reactor, and at the same time, the third reactant enters the second reactor through the fourth inlet, and the obtained oil-in-water emulsion can be solidified to obtain high drug-loaded microspheres. Optional situation 2: the first reactant and the second react...
Embodiment 2
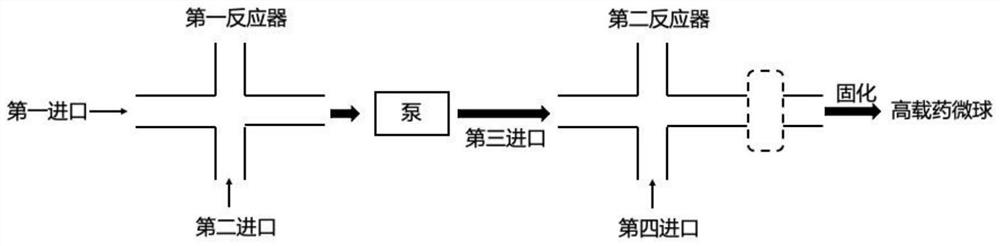
[0051] Such as figure 2 As shown, this embodiment discloses a continuous flow preparation device for highly drug-loaded microspheres. The difference between this embodiment and Embodiment 1 is that there are two second inlets, which are respectively perpendicular to the straight line formed by the first reactor and the first inlet; there are two fourth inlets, which are respectively perpendicular to the second reactor and the third inlet formed straight line.
[0052] The first reactant and the second reactant enter the first reactor through the first inlet and the second inlet respectively, and the obtained mixture of the first reactant and the second reactant enters the second reactor through the third inlet under the action of the pump. In the reactor, at the same time, the third reactant enters the second reactor through the fourth inlet, and the obtained oil-in-water emulsion can be solidified to obtain high drug-loaded microspheres. The exchange of the inlet of the fi...
Embodiment 3
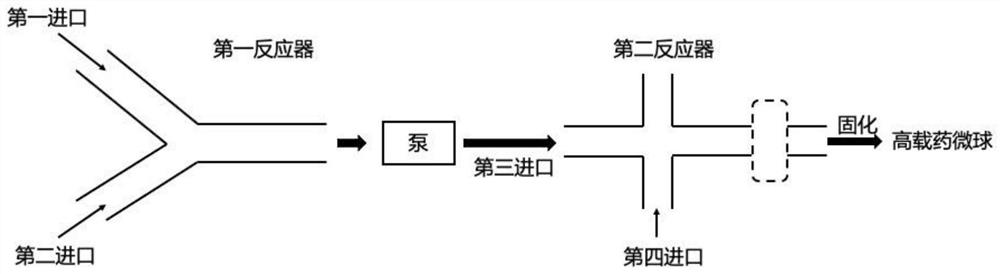
[0054] Such as image 3 As shown, this embodiment discloses a continuous flow preparation device for highly drug-loaded microspheres. The difference between this embodiment and embodiment 2 is that there is one second inlet, the first inlet and the second inlet both form an included angle (0-180 degrees) with the first reactor, and the rest are the same.
[0055] The first reactant and the second reactant enter the first reactor through the first inlet and the second inlet respectively, and the obtained mixture of the first reactant and the second reactant enters the second reactor through the third inlet under the action of the pump. In the reactor, at the same time, the third reactant enters the second reactor through the fourth inlet, and the obtained oil-in-water emulsion can be solidified to obtain high drug-loaded microspheres. The exchange of the inlet of the first reactant and the second reactant into the first reactor does not affect the preparation of high drug-load...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com