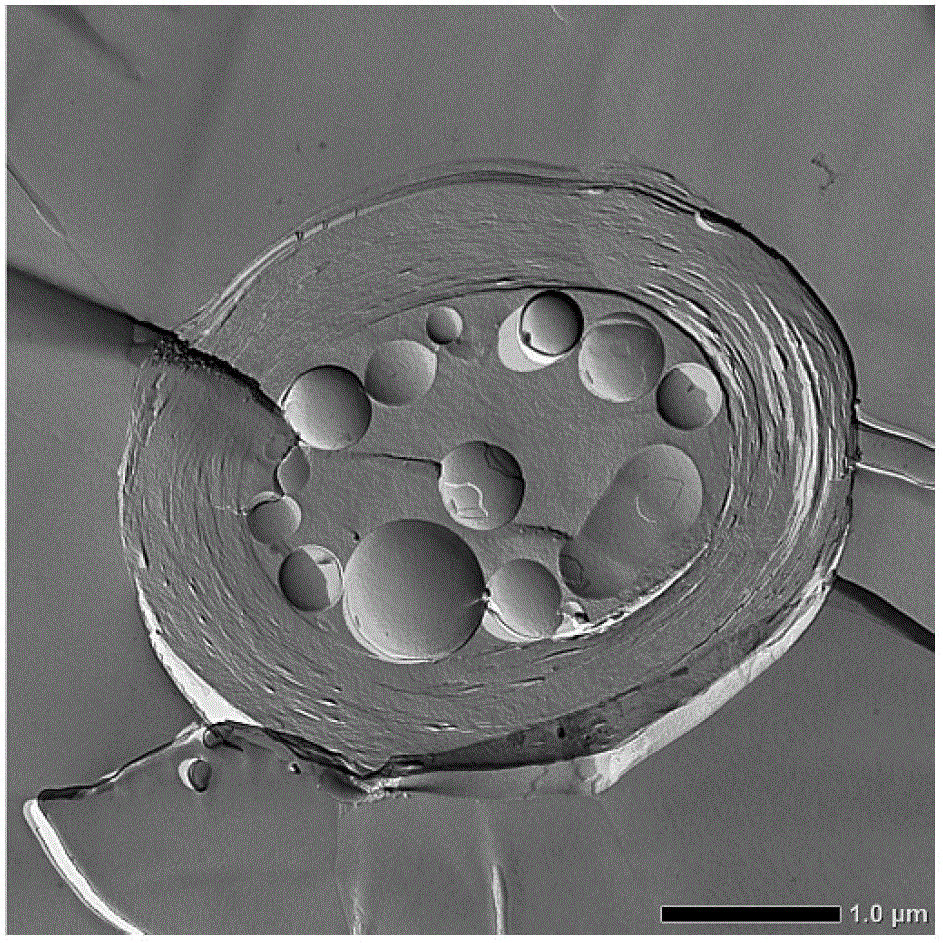
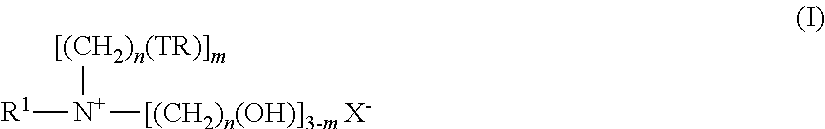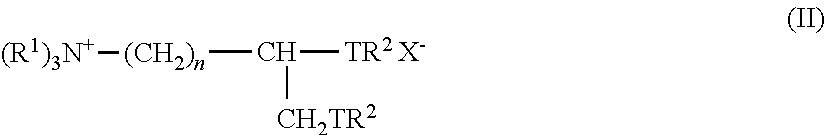Patents
Literature
35results about How to "Efficient encapsulation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
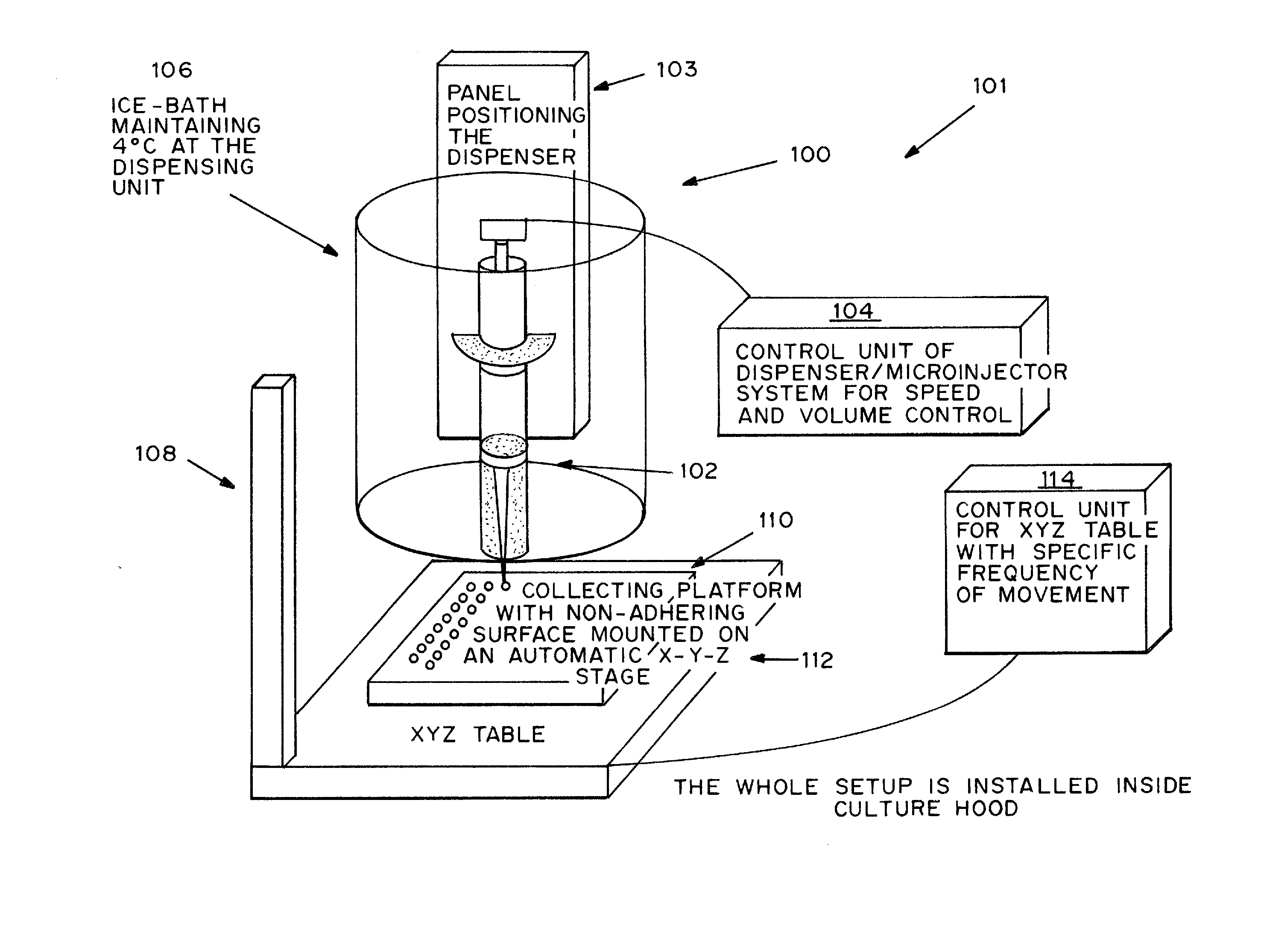
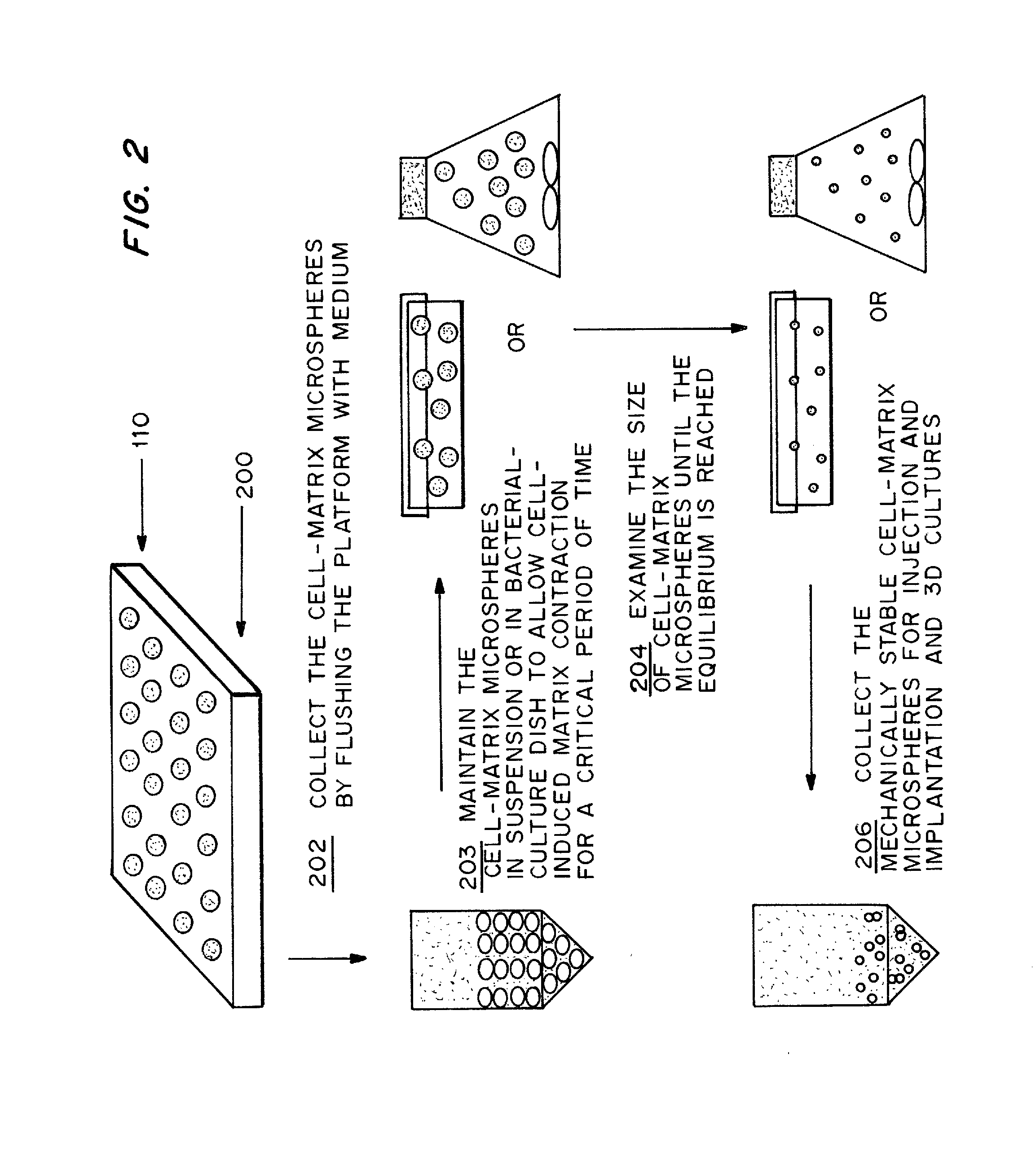
Cell-matrix microspheres, methods for preparation and applications
ActiveUS20080031858A1Increased protein productivityStable cell-matrix microspheresBiocideBioreactor/fermenter combinationsEnzymatic digestionHigh cell
A method has been developed to produce stable cell-matrix microspheres with up to 100% encapsulation efficiency and high cell viability, using matrix or biomaterial systems with poor shape and mechanical stability for applications including cell therapeutics via microinjection or surgical implantation, 3D culture for in vitro expansion without repeated cell splitting using enzymatic digestion or mechanical dissociation and for enhanced production of therapeutic biomolecules, and in vitro modeling for morphogenesis studies. The modified droplet generation method is simple and scalable and enables the production of cell-matrix microspheres when the matrix or biomaterial system used has low concentration, with slow phase transition, with poor shape and mechanical stability.
Owner:VERSITECH LTD
Microparticles
A process for producing parenterally administrable microparticles, in which an at least 20% by weight aqueous solution of purified amylopectin-based starch of reduced molecular weight is prepared, the solution is combined with biologically active substance, an emulsion of starch droplets is formed in an outer phase of polymer solution, the starch droplets are made to gel, and the gelled starch particles are dried. A release-controlling shell is optionally also applied to the particles. Microparticles which essentially consist of said starch, have an amino acid content of less than 50 mug and have no covalent chemical cross-linking.
Owner:PACIRA PHARMA INC
Hyaluronic acid derivative and pharmaceutical composition thereof
ActiveUS20110212901A1Extended stayRetain biological activitySugar derivativesPharmaceutical delivery mechanismMedicinal chemistryHyaluronic acid
The present invention provides a hydrophobic group-introduced hyaluronic acid derivative comprising at least one repeating unit represented by the formula (I):wherein R1, R2, R3, R4, Z, n, Ra, Y, and X1 are as defined in the specification.
Owner:NAT UNIV CORP TOKYO MEDICAL & DENTAL UNIV +1
Microparticles
InactiveUS20020044976A1High yieldEfficient encapsulationPeptide/protein ingredientsGlass/slag layered productsMicroparticlePolymer solution
A process for producing parenterally administrable microparticles, in which an at least 20% by weight aqueous solution of purified amylopectin-based starch of reduced molecular weight is prepared, the solution is combined with biologically active substance, an emulsion of starch droplets is formed in an outer phase of polymer solution, the starch droplets are made to gel, and the gelled starch particles are dried. A release-controlling shell is optionally also applied to the particles. Microparticles which essentially consist of said starch, have an amino acid content of less than 50 mug and have no covalent chemical cross-linking.
Owner:PACIRA PHARMA INC
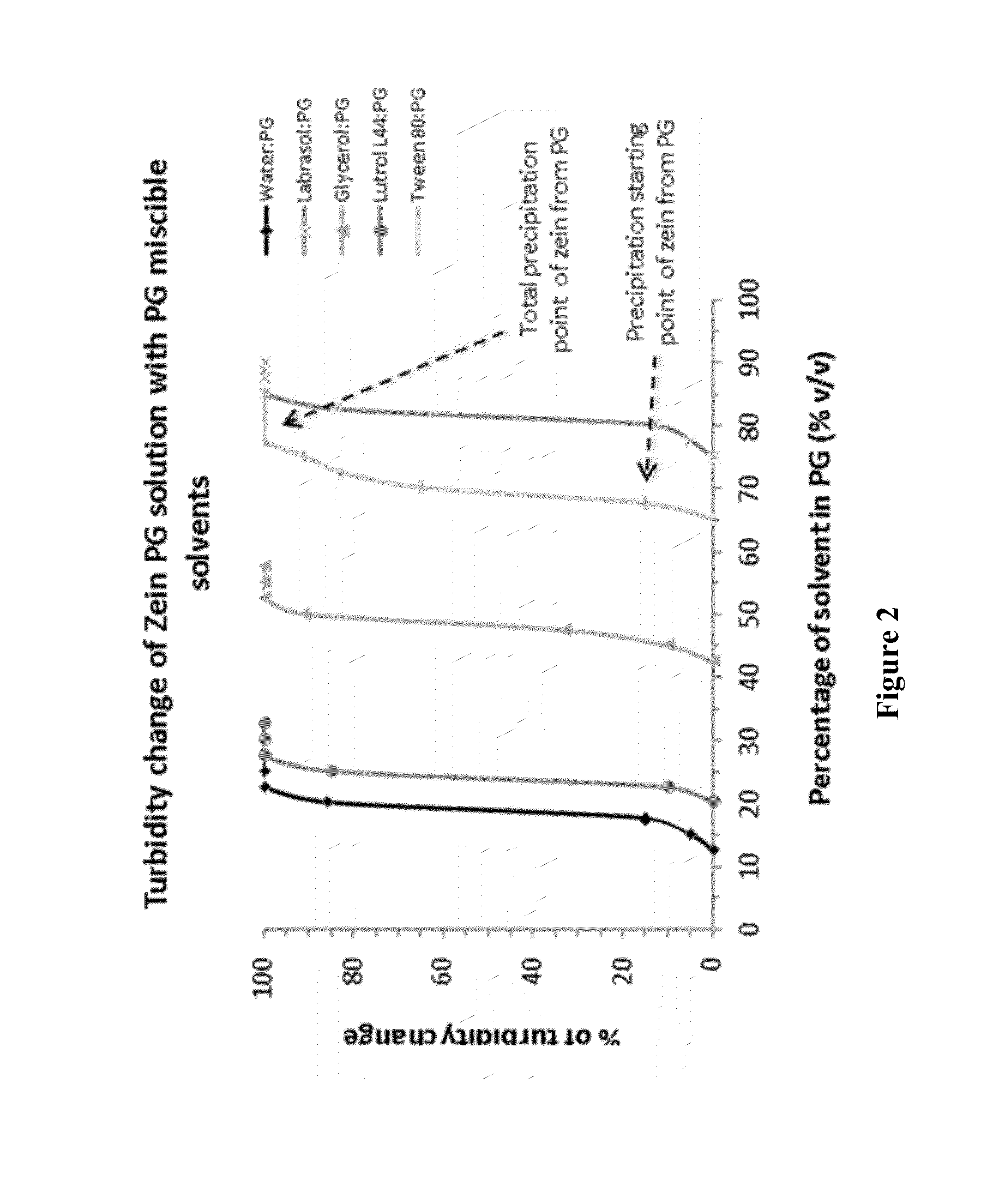
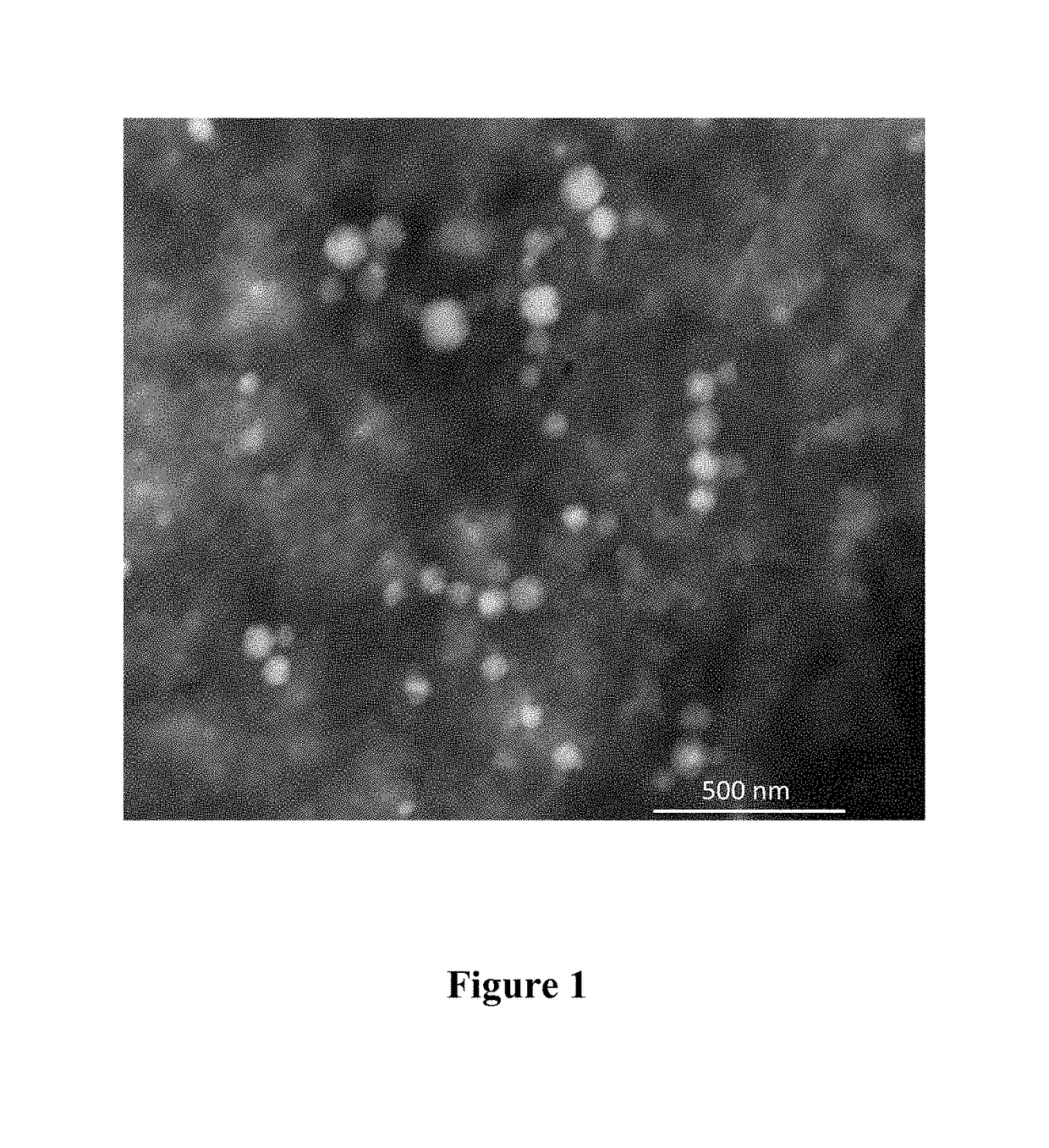
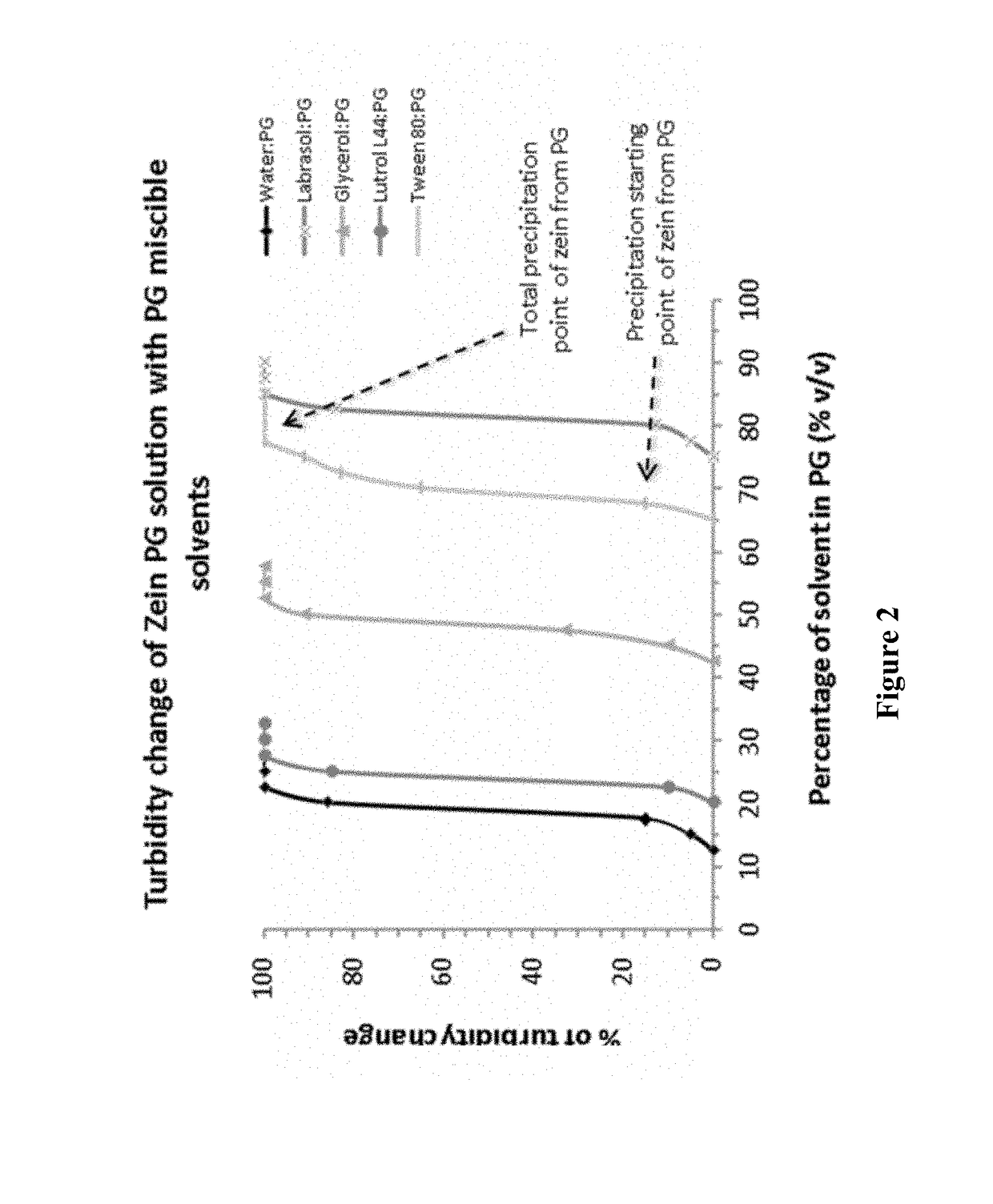
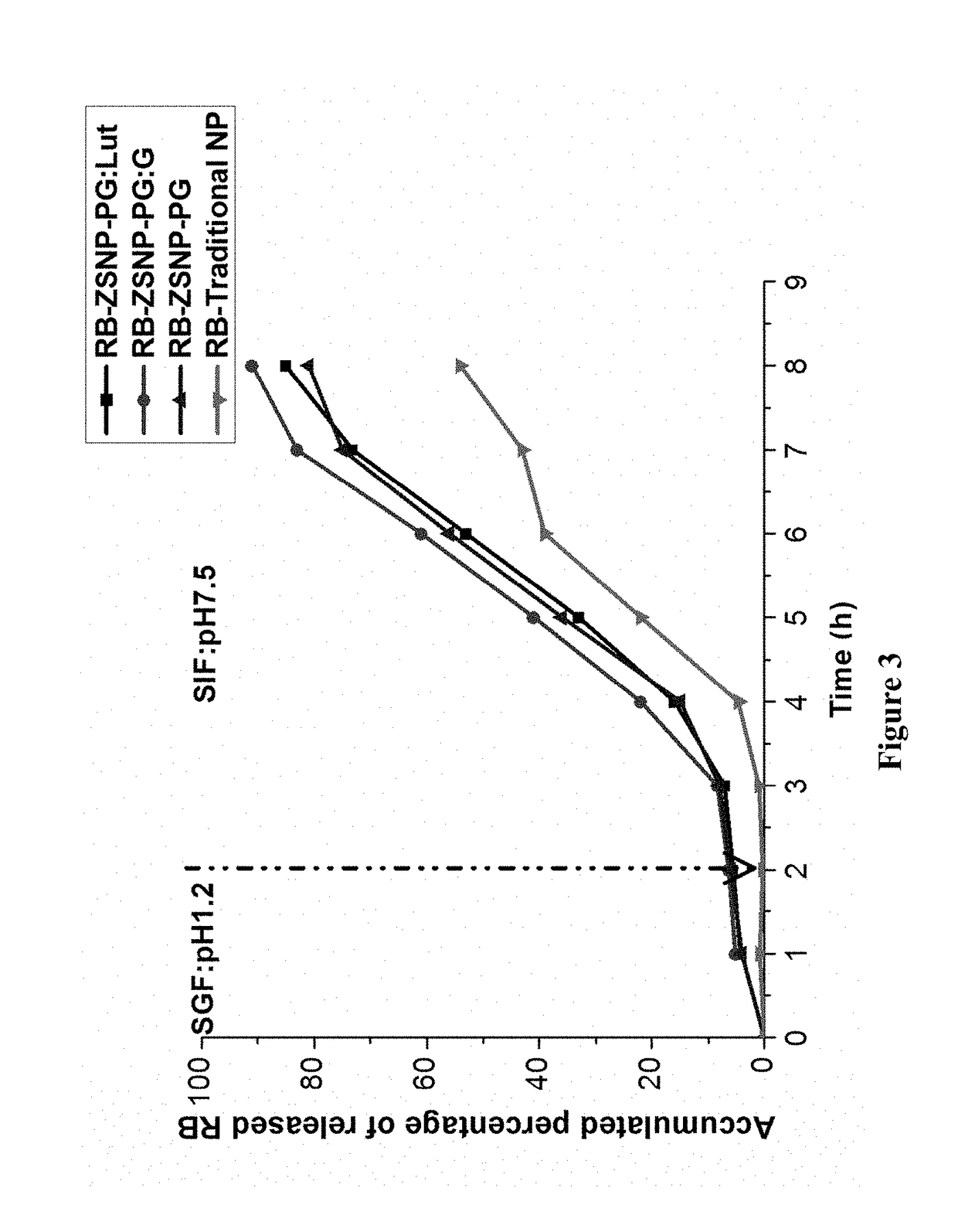
Nanoparticles comprising a vegetable hydrophobic protein and a water miscible non-volatile organic solvent and uses thereof
ActiveUS20150004102A1High yieldSmall sizeAntibacterial agentsPowder deliveryIsopropylene glycolChemistry
The present invention relates to nanoparticles for encapsulating compounds, the preparation and uses thereof, said nanoparticles being based on a vegetable hydrophobic protein, particularly zein, and a water miscible non-volatile organic solvent, particularly propylene glycol. Said nanoparticles can encapsulate or incorporate a product of interest for use in the agricultural, cosmetic, food or pharmaceutical fields.
Owner:BIONANOPLUS
Method for producing flavored particulate solid dispersions
InactiveUS20090301504A1High pHImprove high temperature stabilityTobacco treatmentFood preparationFlavorMoisture
A method for producing flavoring materials consisting of a flavor and a simple matrix material that are GRAS or approved for use in food, and that will be stable under extremely adverse conditions, greater than 50% moisture, high pH, high temperature stability (60° C.), yet be released in the oral cavity; without the use of organic solvents, and with the use of inexpensive materials and unsophisticated equipment.
Owner:SWEDISH MATCH NORTH AMERICA
Fabric conditioners comprising encapsulated active material
ActiveUS20160177241A1Improve retentionHigh retention rateOrganic detergent compounding agentsSoftening compositionsMedicinePolymer
A fabric conditioning composition comprising: (a) at least 8 wt % of a fabric conditioning active; (b) a first capsule containing an active material, wherein the first capsule comprises a cured polymeric wall and a core; and (c) a second capsule containing an active material wherein the second capsule comprises a cured polymeric wall and a core; wherein the first and second capsules differ in properties due to their polymer walls having been made using the same polymer and different cure temperatures, curing times, or a combination thereof.
Owner:CONOPCO INC D B A UNILEVER
Pharmaceutical compositions comprising bisphosponates
InactiveUS20100047306A1Efficient encapsulationEfficient packagingBiocidePowder deliveryDrugProliferative disease
The present invention relates to depot formulations comprising a poorly water soluble salt of a bisphosphonate forming together with one or more biocompatible polymers. The depot formulation may be in the form of microparticles or implants. The depot formulations are useful for the treatment and prevention of proliferative diseases including cancer.
Owner:NOVARTIS AG
Physiologically Active Polypeptide- or Protein-Encapsulating Polymer Micelles, and Method for Production of the Same
ActiveUS20090291130A1Efficient encapsulationEfficient packagingPeptide/protein ingredientsMetabolism disorderChemistryProtein formation
Owner:NANOCARRIER
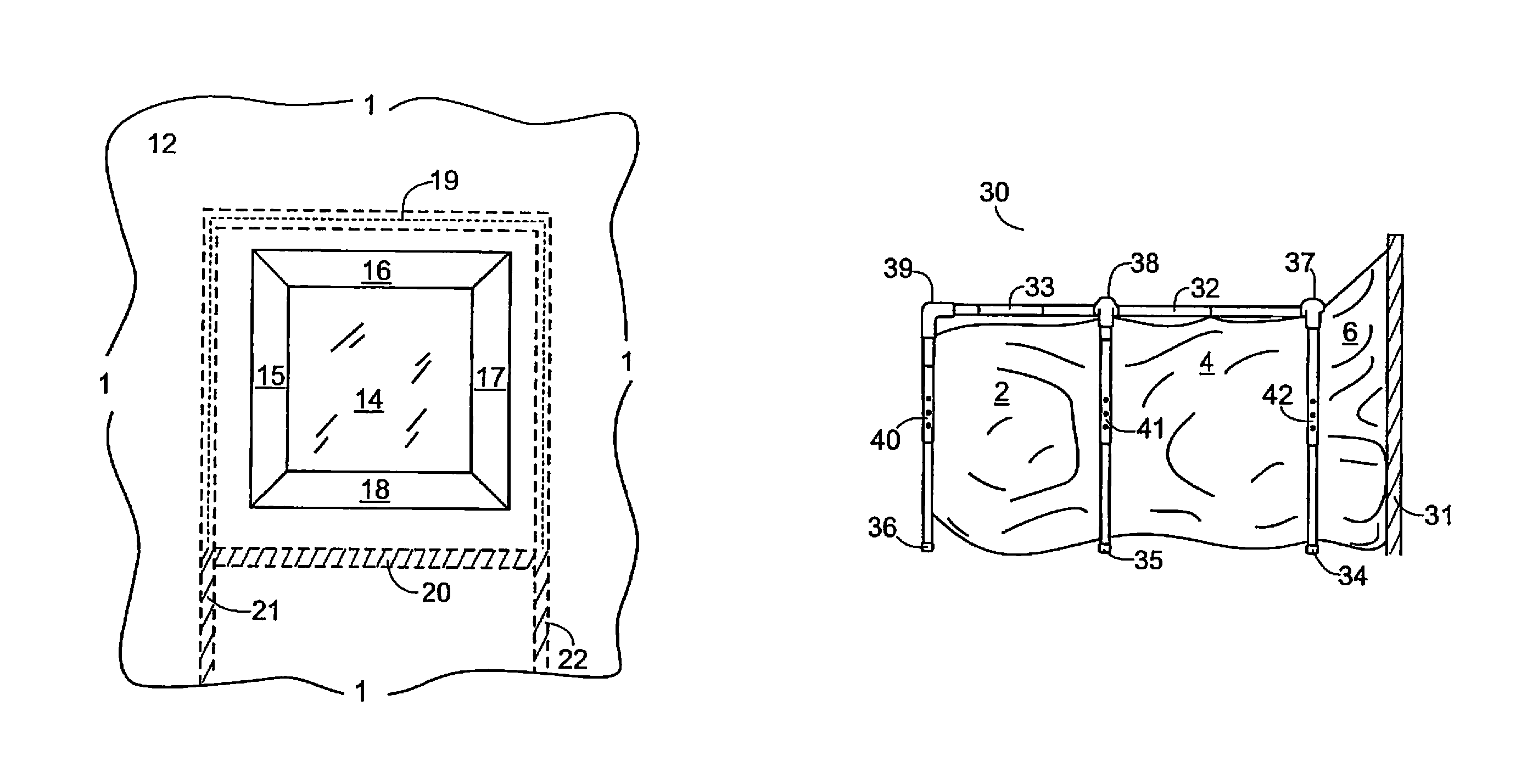
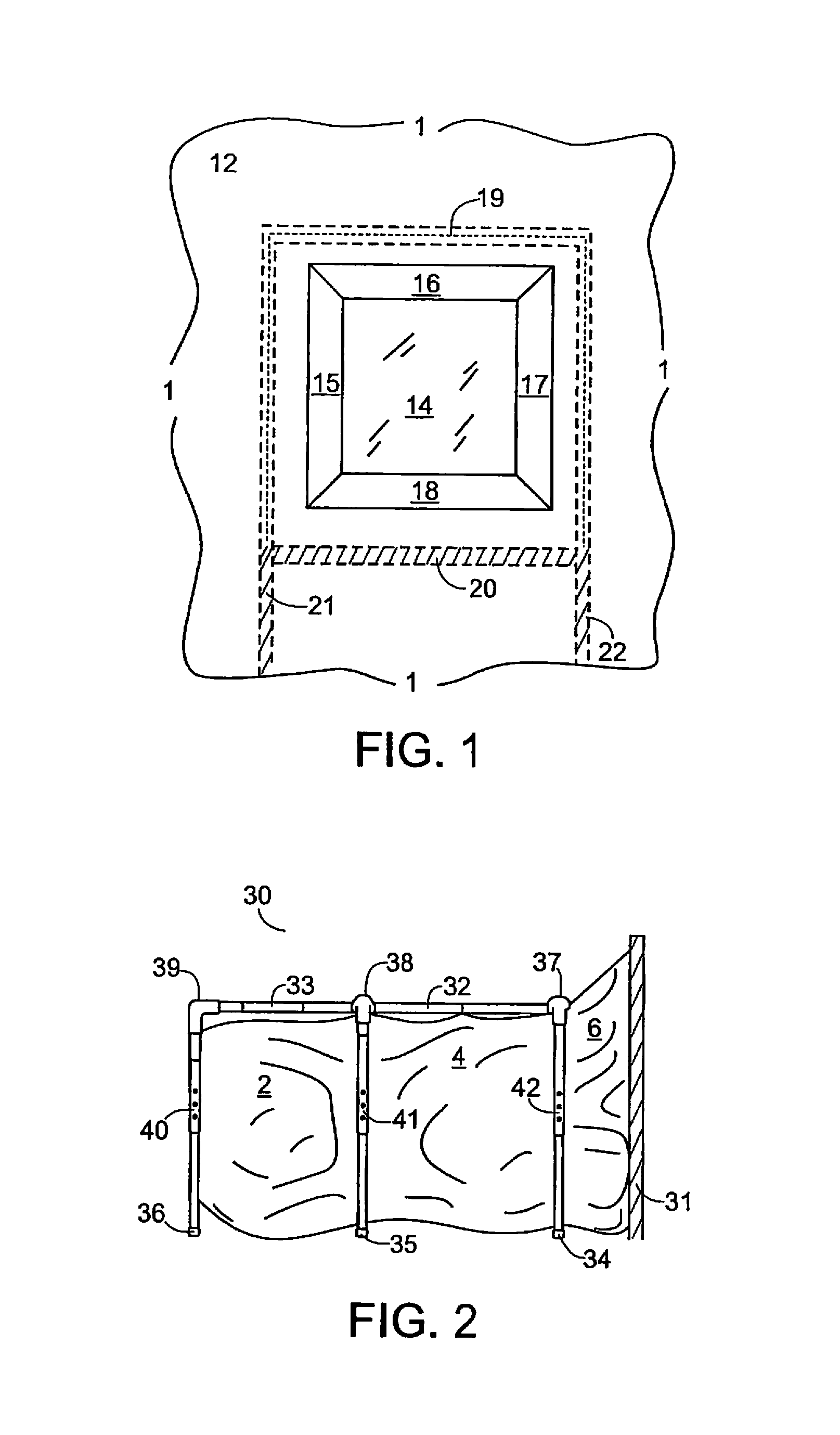
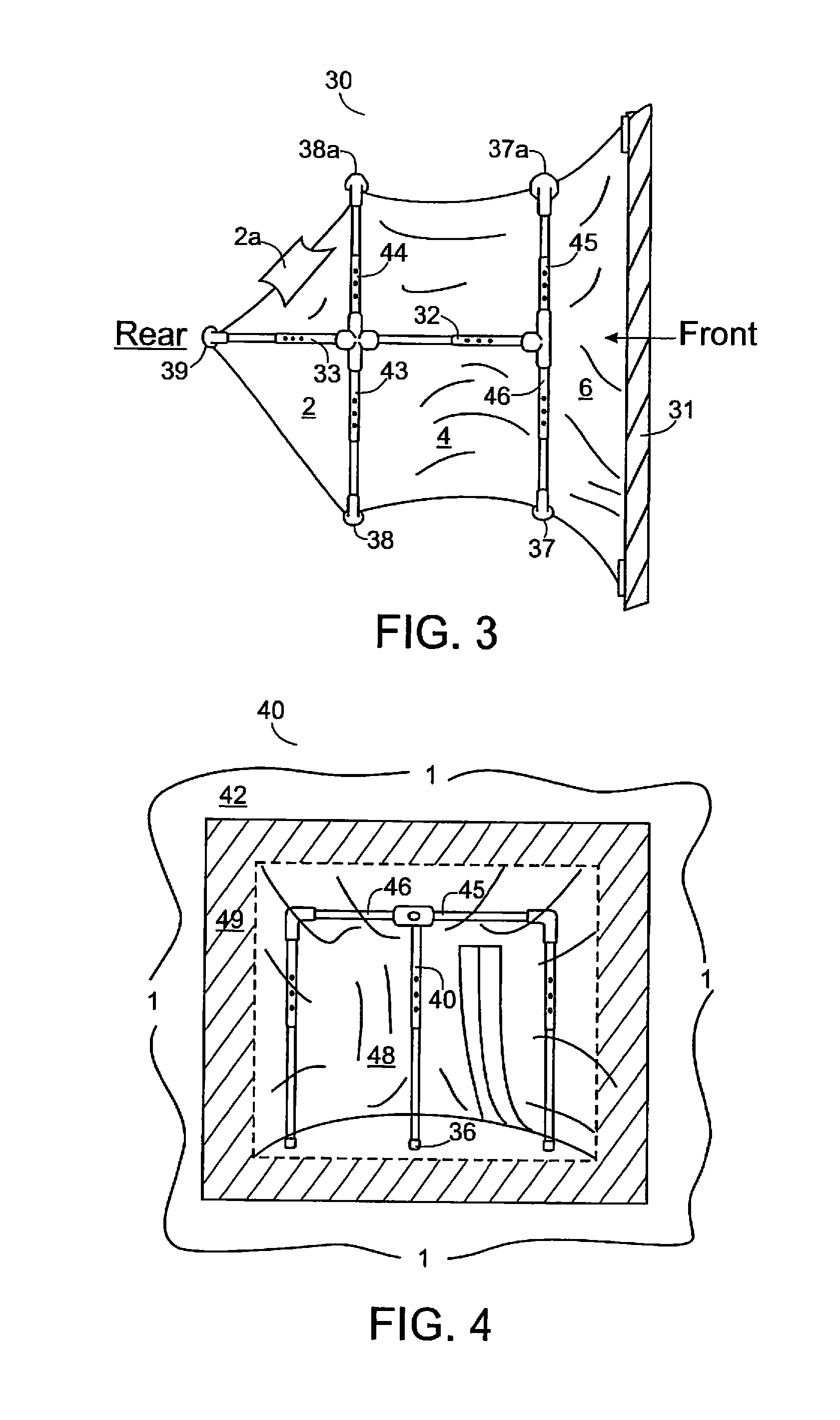
Lead and particulate abatement system
ActiveUS9255422B1Efficient captureEfficient encapsulationTents/canopiesProtective buildings/sheltersParticulatesHazardous substance
An encapsulating system for abating and mitigating contamination from lead-based dust, particles and / or chips in a construction repair, renovation or removal operation and thereby minimizing exposure to lead-based hazardous materials is provided. The apparatus utilizes a framing mechanism supporting an encapsulator container or workbag adapted to surround and seal off a work-area encompassing a contaminated work-piece. The framing mechanism is typically adjustable poles or an adjustable frame apparatus having a plurality of height adjustable vertical legs. The flexible plastic encapsulating container is positioned and supported by the adjustable poles or frame. The container has at least one open-end for surrounding the contaminated work-piece, and the open-end of the container is adhered to the work-area and substantially surrounds and seals off the contaminated work-piece from the interior portions of the work site.
Owner:ENCAPSULATOR LLC
Organic nanotube having hydrophobized inner surface, and encapsulated medicinal agent prepared using the nanotube
InactiveUS9018156B2Stably maintaining tube shapeGood dispersionMaterial nanotechnologyPeptide/protein ingredientsCarboxylic acidPharmaceutical Substances
Provided is an organic nanotube having a hydrophobized inner surface, formed by molecules including an asymmetric bipolar lipid molecule represented by the following General Formula (1) and a derivative thereof represented by the following General Formula (2), wherein the organic nanotube has a hydrophilized outer surface and a hydrophobized inner surface of a hollow cylinder and is formed by binary self-assembly, the organic nanotube encapsulates a hydrophobic guest in the hollow cylinder, has a function of refolding a denatured protein, and has a function of sustainably-releasing a hydrophobic drug according to the change in hydrophobicity of the inner surface of the tube or external stimulus,In Formulas (1) and (2), wherein the same symbols have the same meanings, G is a 1-glucopyranosyl group or 2-glucopyranosyl group, and n is an integer of 12 to 22. Particularly, an asymmetric bipolar lipid molecule and an ester thereof respectively represented by general formulae (1) and (2) wherein n is an integer of 18 to 22, both of Z1 and Z2 are single bonds, Y is Gly, m(s) is the same or different integer of 3 to 6, X is OH, and R is a methoxy group, an ethoxy group, or a benzyloxy group are novel substances, and can form a carboxylic acid based asymmetric nanotube by single component self-assembly or binary self-assembly.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Process for producing wet ribavirin pellets
InactiveUS20050075297A1Efficient encapsulationHeat generationBiocideCarbohydrate active ingredientsPharmaceutical preservativesEngineering
A process for producing wet ribavirin pellets is provided in order to make pharmaceutical dosages of ribavirin. The process is particularly useful as an alternative method for preparing pharmaceutical dosages of ribavirin that reduces the amount of ribavirin dust that is produced during the manufacturing process and allows for greater control of dissolution rates. According to the preferred embodiments, this method is accomplished through mixing ribavirin with at least one excipient into a uniform mixture, forming the mixture into a granulated mass by adding a wetting agent, shaping said granulated mass into soluble particles and drying the flowable particles. The process enables Ribavirin pharmaceutical pellets to be mixed with a binder and disintegrant to form a uniform mixture.
Owner:KADMON PHARMA LLC
Loaded micropeptide nano liposome and preparation, application and use method thereof
InactiveCN108904369AEfficient encapsulationPromote absorptionCosmetic preparationsToilet preparationsFreeze-dryingCholesterol
The invention relates to loaded micropeptide nano liposome and a preparation, an application and use method thereof, which belong to the technical field of cosmetic. The method utilizes lecithin and cholesterol to prepare nano liposome; and uses the micropeptide, sodium hyaluronate and a gynostemma pentaphyllum extract to prepare micropeptide-encapsulated liposome; the micropeptide-encapsulated liposome is sheared by a homogenizer to prepare a nano-liposome solution; the soluble proteoglycan, glucose, sodium hyaluronate, mannose, nicotinamide and glucuronic acid are added to the nano-liposomesolution, and the loaded micropeptide nano liposome is obtained after freeze drying. The method has an unique packaging method and fully utilizes the comprehensive performance of micropeptide, sodiumhyaluronate and the gynostemma pentaphyllum extract, maximizes the anti-aging effect, at the same time, the encapsulation properties of liposome are used to keep the activity of micropeptide, and convenient preservation and carrying can be realized.
Owner:YANGZHOU MENGXIAO BIOLOGICAL ENG CO LTD
Nanoparticles comprising a vegetable hydrophobic protein and a water miscible non-volatile organic solvent and uses thereof
ActiveUS9974753B2Small sizeHigh yieldAntibacterial agentsOrganic active ingredientsOrganic solventCompound (substance)
The present invention relates to nanoparticles for encapsulating compounds, the preparation and uses thereof, said nanoparticles being based on a vegetable hydrophobic protein, particularly zein, and a water miscible non-volatile organic solvent, particularly propylene glycol. Said nanoparticles can encapsulate or incorporate a product of interest for use in the agricultural, cosmetic, food or pharmaceutical fields.
Owner:BIONANOPLUS
Thermosetting silicone resin sheet and method for producing the same, and light-emitting apparatus using the thermosetting silicone resin sheet and method for producing the same
ActiveUS9117978B2Readily be laminatedMaintain stabilityLayered productsSolid-state devicesPhosphorSemi solid
The present invention provides a thermosetting silicone resin sheet having a phosphor-containing thermosetting silicone resin layer formed in the form of an LED device and a method for producing the same, and a light-emitting apparatus using the thermosetting silicone resin sheet and a method for producing the same. The present invention was accomplished by the phosphor-containing thermosetting silicone resin sheet comprising a substrate film and a phosphor-containing thermosetting silicone resin layer that is a plastic solid or a semi-solid at room temperature composed of a single layer having no adhesive layer, formed by printing and molding a phosphor-containing thermosetting silicone resin composition on the substrate film in the form of an LED device.
Owner:SHIN ETSU CHEM CO LTD
Double-network hydrogel shell polycystic core structure trichoderma harzianum agent
ActiveCN112111412AEfficient encapsulationReduce lossesFungiMicroorganism based processesFungicidePolyvinyl alcohol
The invention belongs to the field of microbial fertilizers, and particularly relates to a double-network hydrogel shell polycystic core structure trichoderma harzianum agent as well as a preparationmethod and application thereof. The preparation method comprises the following steps that trichoderma harzianum, bacterial cellulose, soluble starch, calcium carbonate and other powder are uniformly mixed to prepare a fungicide core layer; a polyvinyl alcohol film is attached to the surface of the fungicide nuclear layer, so that the fungicide nuclear layer is isolated from the external environment; then coating with a nutrition synergist represented by glucose, ammonium sulfate, monopotassium phosphate and the like is peformed; and finally, a polyvinyl alcohol-glycerol / gelatin double-networkhydrogel shell layer on the surface of a nutrition synergistic layer. According to the method, the trichoderma harzianum agent with low bacterial powder encapsulation amount and high viable bacteriumrelease amount can be obtained.
Owner:江西华威科技有限公司
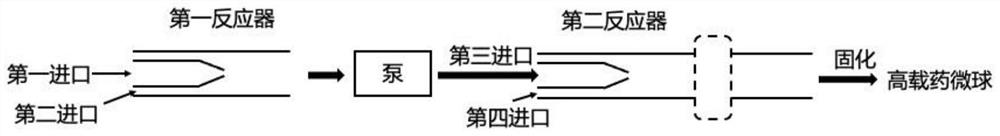
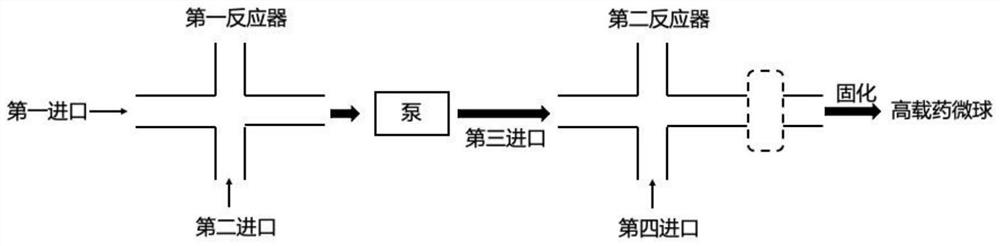
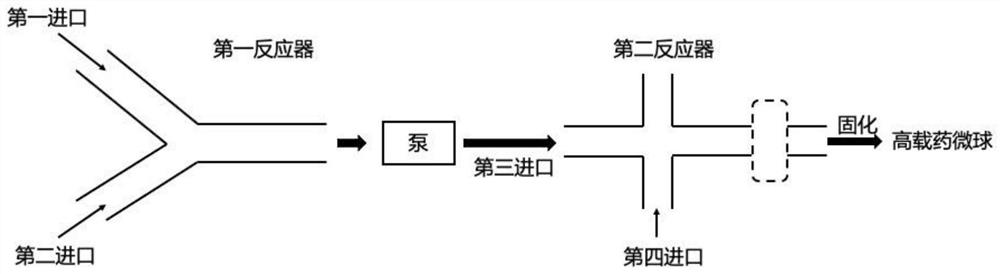
Continuous flow preparation method of high drug-loading microspheres
PendingCN114146647APrevent leakageAvoid qualitative differencesPharmaceutical non-active ingredientsMicroballoon preparationDrugs solutionMicrosphere
The invention belongs to the technical field of pharmaceutical preparations, and discloses a continuous flow preparation method of high-drug-loading microspheres. The method comprises the following steps: precipitating an active drug solution by using a poor solvent in which a microsphere matrix material is dissolved to form nanoparticles, directly taking the mixture as an oil phase, mixing the oil phase with a water phase to prepare oil-in-water emulsion droplets, and curing to obtain the high-drug-loading microspheres. The nanoparticles are directly wrapped in one step in continuous flow preparation, the preparation process of the microspheres is simplified, and the application value is high. The prepared high-drug-loading microsphere comprises nanoparticles composed of active drug components and a microsphere matrix material used for controlling drug release, and the particle size is 1-2000 [mu] m; the mass of the active drug component accounts for 5-80% of the mass of the whole microsphere, and the encapsulation efficiency of the active drug is 5-100%. The microspheres prepared through a continuous flow method are high in encapsulation efficiency and drug loading capacity and free of burst release, the treatment efficiency can be effectively improved, and toxic and side effects and adverse reactions can be reduced.
Owner:CHINA PHARM UNIV
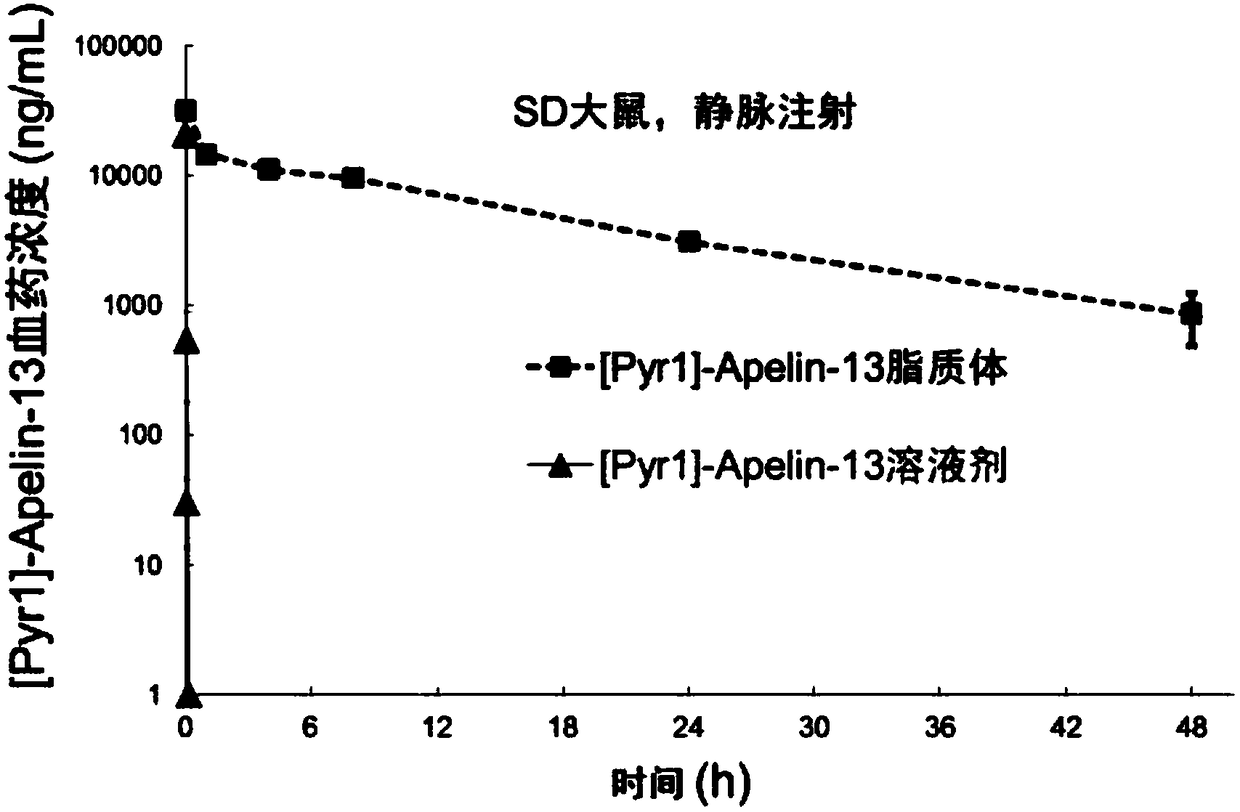
Apelin liposome and preparation method thereof
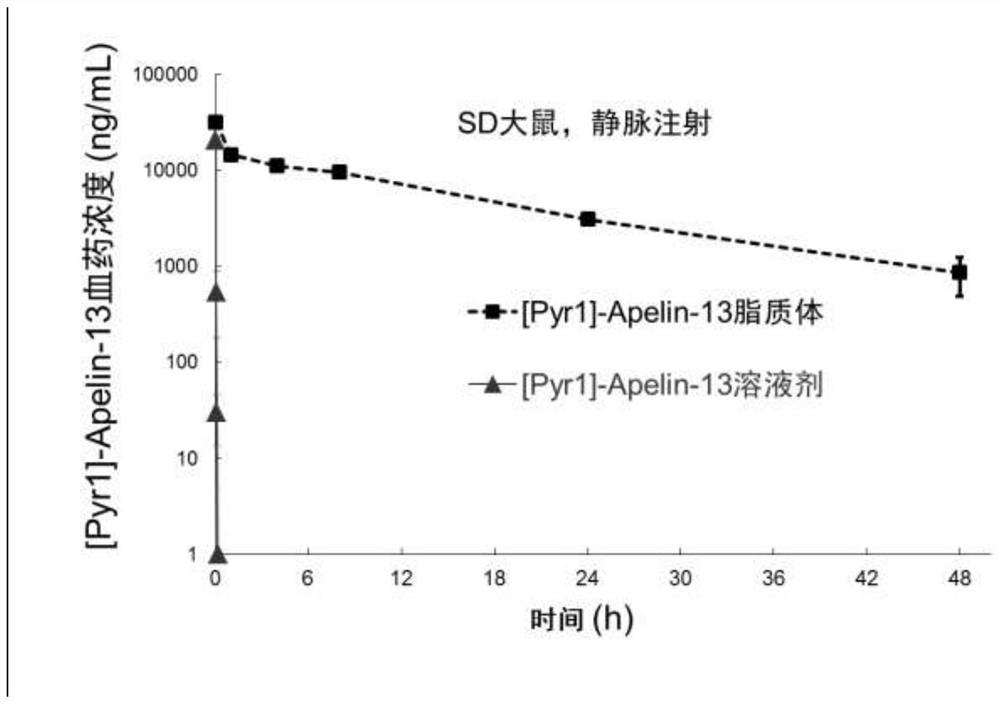
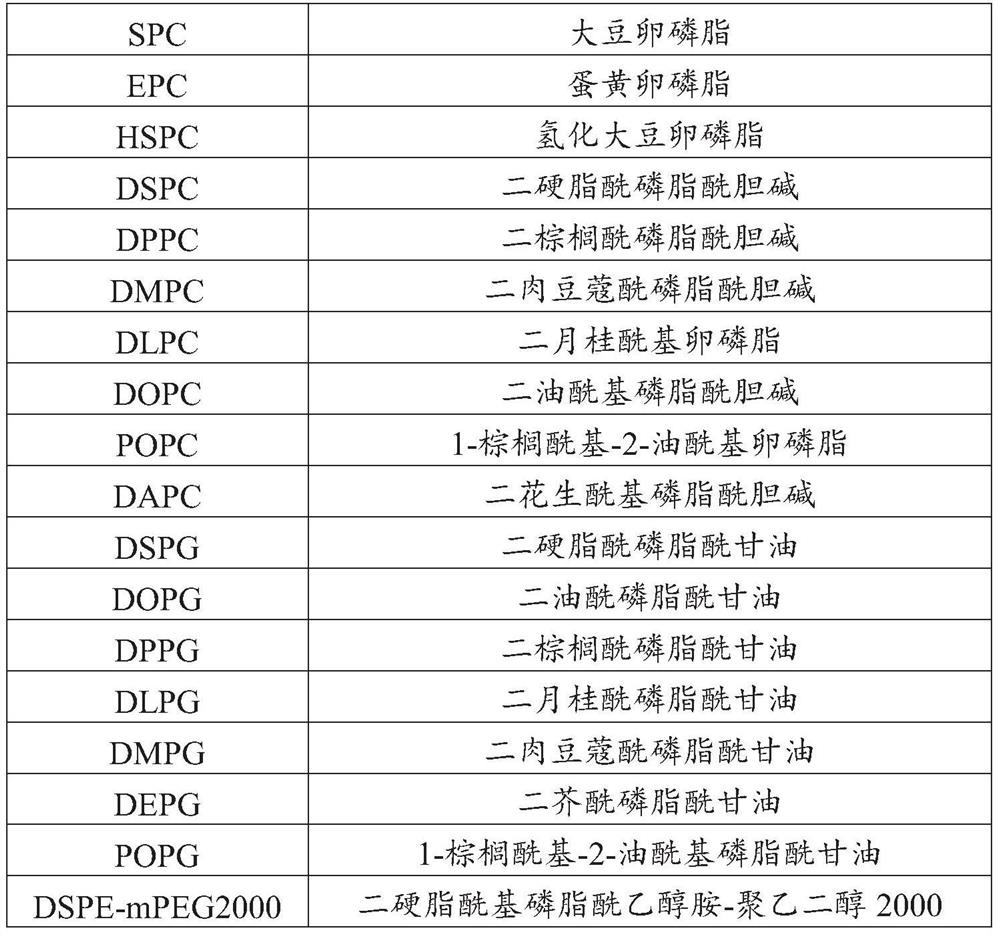
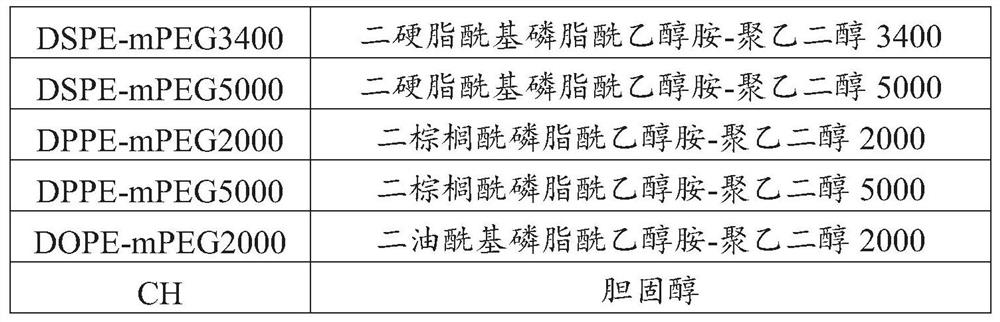
ActiveCN108159401AEfficient encapsulationExtended half-lifePeptide/protein ingredientsPharmaceutical non-active ingredientsHalf-lifeChemistry
The invention provides an Apelin liposome and a preparation method thereof. The Apelin liposome comprises Apelin, phosphatidylglycerol, phosphatidylcholine, cholesterol, phosphatidyl ethanolamine-polyethylene glycol derivatives, and the molar ratio of Apelin to phosphatidylglycerol is (1:2)-(1:10). The Apelin liposome achieves highly efficient encapsulation of Apelin, the encapsulation efficiencyis more than 90%, and meanwhile the half-life period of Apelin in vivo is significantly prolonged.
Owner:KUNMING JIDA PHARMA
Virus-like particles and use thereof
PendingUS20210269790A1Efficient packagingHigh activityOrganic active ingredientsHydrolasesDimerProtein target
A virus-like particle encapsulating a target protein is provided. The virus-like particle contain a Gag protein, and the Gag protein forms a dimer with the target protein.
Owner:KYOTO UNIV
Liposome composition and process for production thereof
ActiveCN103153285AEfficient encapsulationAchieve long-term sustained releasePharmaceutical non-active ingredientsLiposomal deliveryDrugIon
The purpose of the present invention is to provide a liposome composition into which a drug can be introduced in a high encapsulation amount, and which has sustained release properties to such an extent that the effective concentration can be maintained at a clinically satisfactory level, and which is suitable for subcutaneous administration or the like. This liposome composition comprises a first liposome which has an outer membrane composed of a multilayered lipid bilayer membrane and multiple second liposomes which are accommodated in a first liposome inner region that is defined by the outer membrane and each of which has an outer membrane composed of a multilayered lipid bilayer membrane, wherein the liposome composition has second liposome inner regions each defined by the outer membrane of each of the second liposome, and wherein ion gradient is formed at least between each of the second liposome inner regions and the outside of the first liposome.
Owner:TERUMO KK
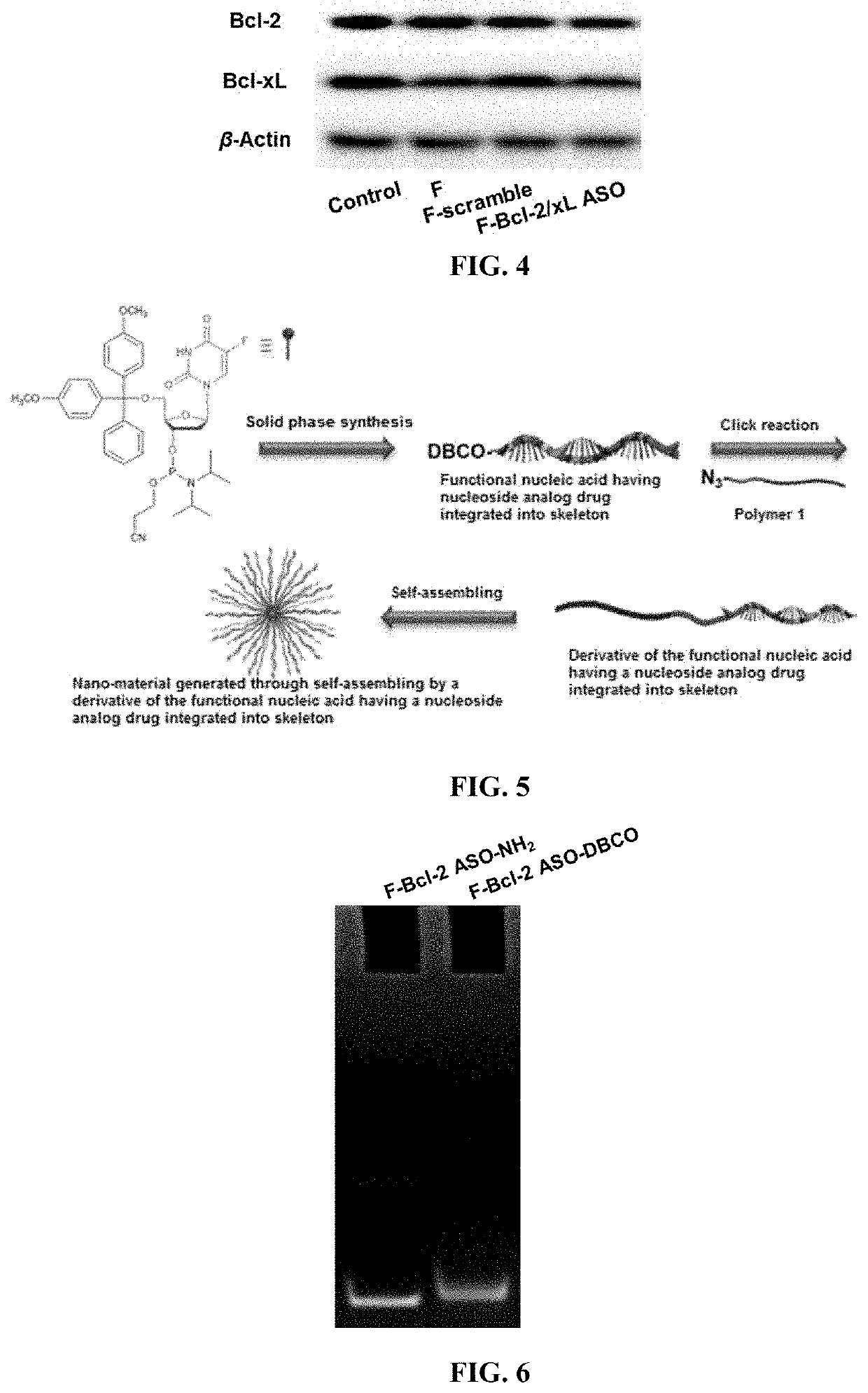
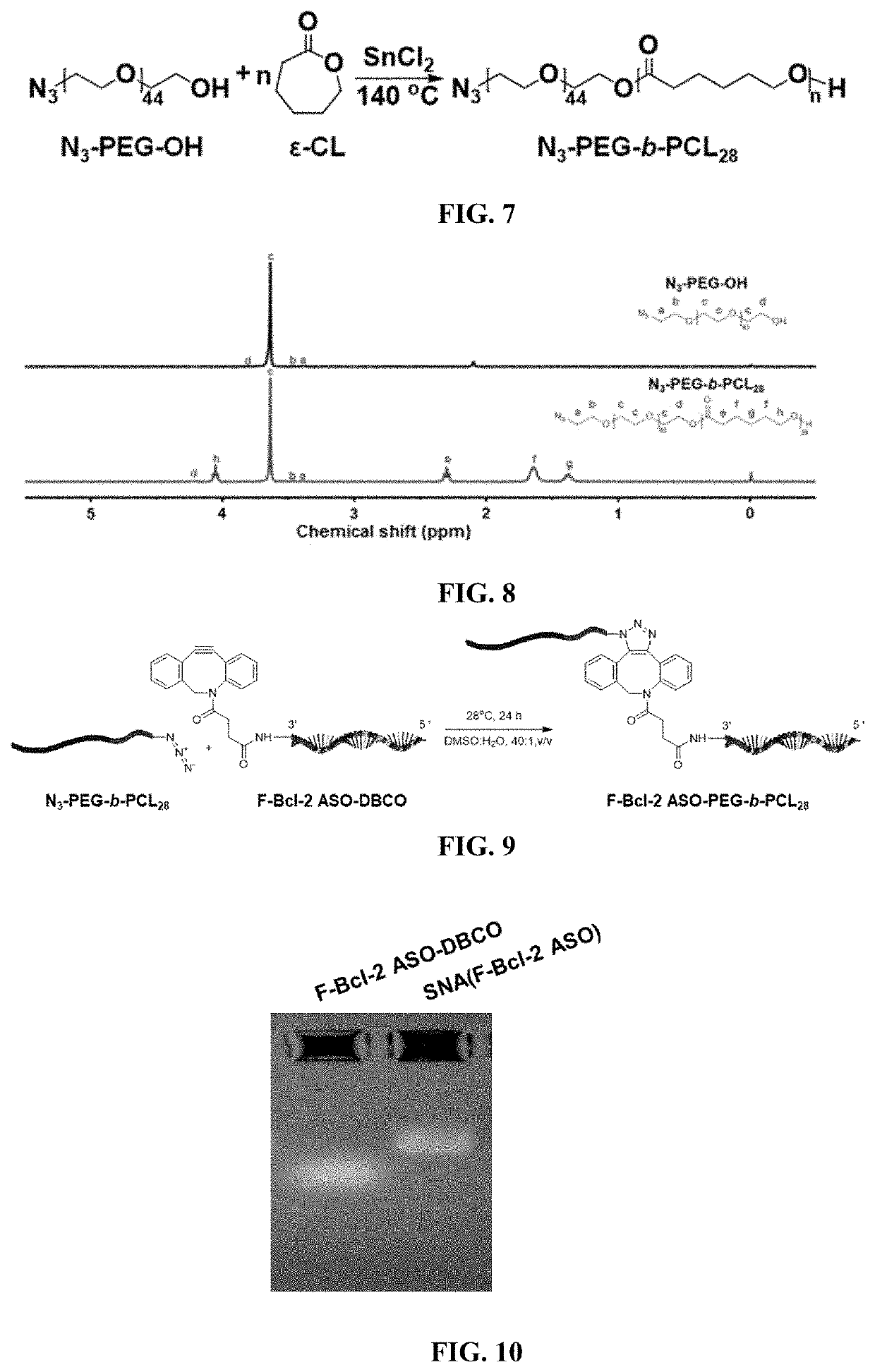
Functional nucleic acid having nucleoside analog drug integrated into skeleton, derivative and use thereof
PendingUS20210196827A1Precise adjustment of ratioMaximize the effectPharmaceutical non-active ingredientsEmulsion deliveryBULK ACTIVE INGREDIENTChemo therapy
The application discloses a functional nucleic acid having nucleoside analog drug integrated into skeleton, a derivative, and preparation methods thereof wherein the derivative is obtained by conjugating or self-assembling the functional nucleic acid having nucleoside analog drug integrated into skeleton with one of a polymer, a hydrophobic molecule, and a transfection reagent. Compared with the prior art, the functional nucleic acid having nucleoside analog drug integrated into skeleton and the derivative thereof can efficiently enter cells and be used to regulate genes; subsequently, the functional nucleic acid having nucleoside analog drug integrated into skeleton can be degraded by nuclease and release active ingredient of the nucleoside analog drug, thus playing a role in chemotherapy. Hence, the functional nucleic acid having nucleoside analog drug integrated into skeleton and the derivative thereof can simply and efficiently realize a combination therapy of gene therapy and chemotherapy, and a complex synthesis procedure is avoided.
Owner:SHANGHAI JIAO TONG UNIV
A double network hydrogel shell polycystic core structure Trichoderma harzianum fungus
ActiveCN112111412BEfficient encapsulationReduce lossesFungiMicroorganism based processesBiotechnologyPolyvinyl alcohol
The invention belongs to the field of microbial bacterial fertilizer, and in particular relates to a trichoderma harzianum fungus with a double-network hydrogel shell and multi-cystic core structure, a preparation method and application thereof. In the present invention, Trichoderma harzianum, bacterial cellulose, soluble starch, calcium carbonate and other powders are evenly mixed to form a bacterial agent core layer; a polyvinyl alcohol film is attached to the surface of the bacterial agent core layer to isolate the bacterial agent core layer from the external environment; Subsequently, the nutritional synergist represented by glucose, ammonium sulfate, potassium dihydrogen phosphate, etc. is coated; finally, a polyvinyl alcohol-glycerol / gelatin double network hydrogel shell is formed on the surface of the nutritional synergistic layer. The method can obtain the Trichoderma harzianum fungus agent with low encapsulation amount of bacterial powder and high bacterial activity.
Owner:江西华威科技有限公司
Apelin liposome and preparation method thereof
ActiveCN108159401BEfficient encapsulationExtended half-lifePeptide/protein ingredientsPharmaceutical non-active ingredientsCholesterolPolyethylene glycol
The present invention provides a kind of Apelin liposome and preparation method thereof, this Apelin liposome comprises Apelin, phosphatidylglycerol, phosphatidylcholine, cholesterol, phosphatidylethanolamine-polyethylene glycol derivative, and Apelin and phosphatidylglycerol The molar ratio is 1:2~1:10. The Apelin liposome of the invention realizes high-efficiency encapsulation of Apelin, with an encapsulation rate of more than 90%, and at the same time significantly prolongs the half-life of Apelin in a living body.
Owner:KUNMING JIDA PHARMA
Liposome composition and its production method
ActiveCN103153285BEfficient encapsulationAchieve long-term sustained releasePharmaceutical non-active ingredientsLiposomal deliveryPharmaceutical drugLipid Body
The purpose of the present invention is to provide a lipid composition that can import drugs with high sealing, and clinically maintains a sufficiently effective concentration, suitable for subcutaneous administration.The lipid composition of the present invention has the first lipid body and multiple second lipids, which has an outer membrane formed by the multi -layer lipid double layer; the second lipid body containmentIn the internal area of the first lipid body defined by the outer membrane, and has an outer membrane formed by multi -layer lipid double layers, the lipid composition hasThe internal area of the dimension, and at least the internal area of the second lipid body forms an ion gradient between the outer part of the first lipid body.
Owner:TERUMO KK
Curcumin microencapsulation method
InactiveCN111481524AImprove stabilityEfficient encapsulationMetabolism disorderAntipyreticAnti-inflammatoryGlycerol
The invention discloses a curcumin microencapsulation method which comprises the following steps: dissolving curcumin in a solvent consisting of ethyl acetate and ethanol with the weight being 8-10 times that of curcumin, then adding tea saponin, lactic acid fatty glyceride and tripolyglycerol monostearate, heating, and uniformly stirring to obtain a core material solution; the preparation methodcomprises the following steps: taking phlorizin, mogroside V, rhamnolipid and xanthan gum as wall materials, adding water of which the weight is 10-15 times that of the wall materials, uniformly stirring, and heating to 60-70 DEG C to obtain a capsule material solution; adding the core material solution into the capsule material solution according to a mass ratio of 1: (2-4), and adding into a homogenizer for homogenizing treatment to obtain a mixed solution; and sending the mixed solution into a granulation sprayer for spray drying, and cooling to room temperature to obtain the curcumin microcapsules. According to the method disclosed by the invention, the curcumin is microencapsulated, so that efficient encapsulation of the curcumin can be effectively realized, the stability of the curcumin is improved, the storage period is prolonged, and the water solubility of the curcumin is improved; the medicine effects of oxidation resistance, blood sugar reduction, inflammation resistance andthe like of the curcumin microcapsules can also be enhanced, and the bad taste of curcumin is hidden.
Owner:右江民族医学院
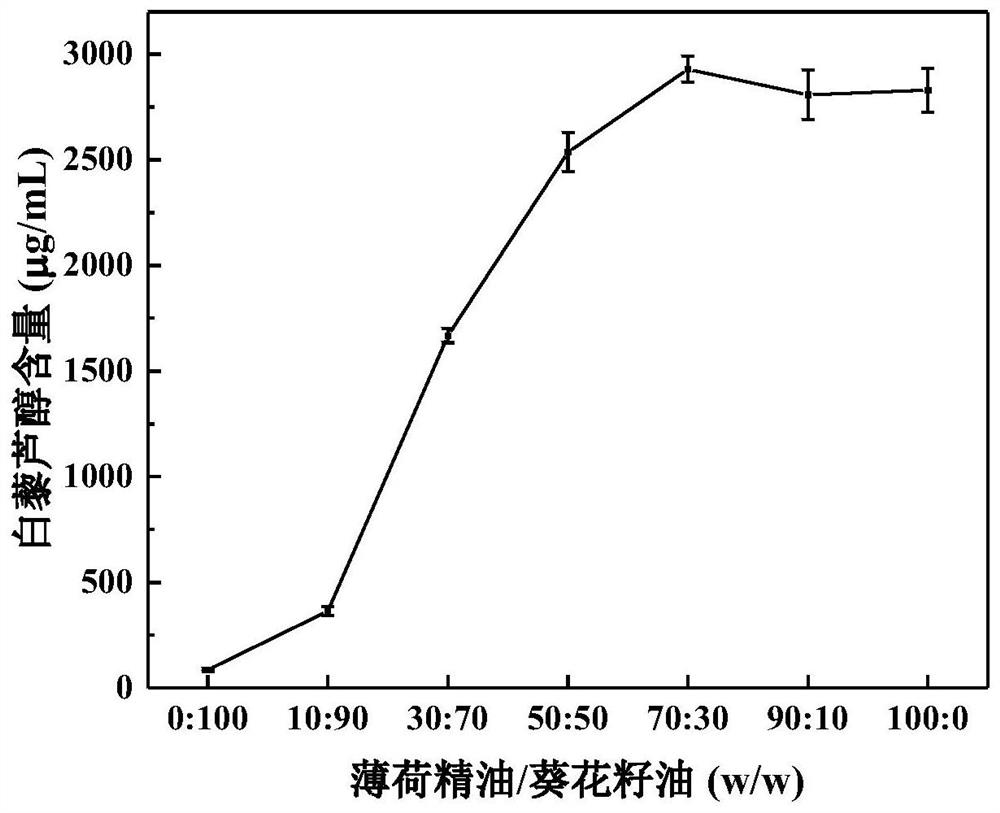
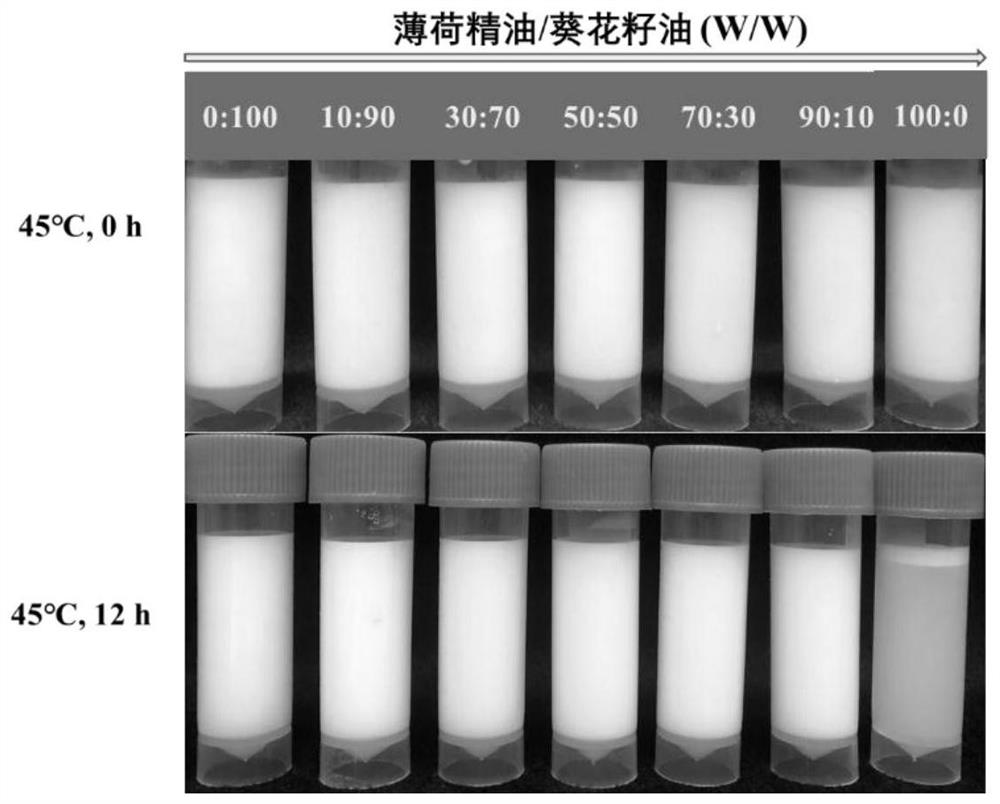
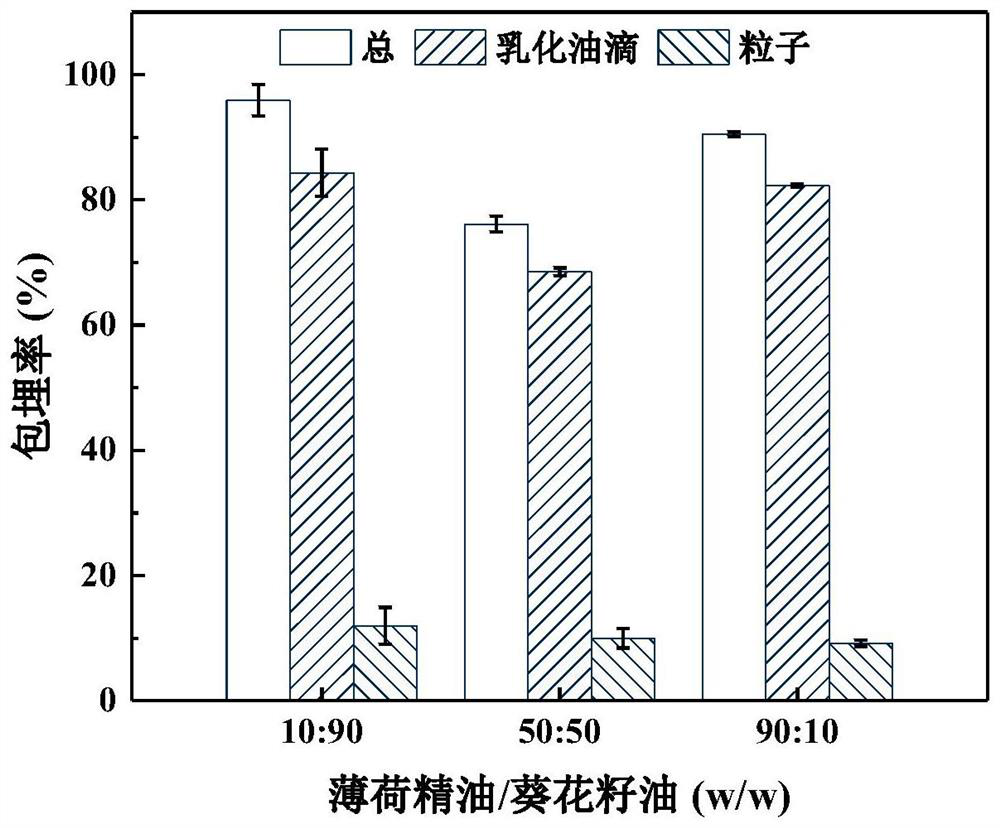
Emulsion for efficiently encapsulating resveratrol and application thereof
ActiveCN113180241AImprove solubilityEfficient encapsulationFood ingredient as emulsifierBiotechnologySodium Caseinate
The invention discloses an emulsion for efficiently encapsulating resveratrol and application of the emulsion, and belongs to the technical field of food processing. The preparation method comprises the following steps: firstly, screening mint essential oil (PO) as a green dispersant of resveratrol, preparing a mixture of PO and sunflower seed oil in any proportion as an internal oil phase, taking a sodium caseinate aqueous solution as a continuous phase, and preparing the resveratrol-containing nano-emulsion through a two-step emulsification process of high-speed shearing and high-pressure homogenization. The nanoemulsion provided by the invention has good storage stability. When the PO content is 50% or above, the retention rate of resveratrol reaches 80% or above after storage at 45 DEG C for 60 days, and the resveratrol can be well protected. The emulsion does not introduce any organic reagent, is simple and convenient to operate and easy to industrialize, and has the advantage of green production. Meanwhile, the emulsion disclosed by the invention is also applicable to encapsulation and protection of fat-soluble functional factors, and has the advantage of wide application range.
Owner:JIANGNAN UNIV
CSP manufacturing process
A CSP manufacturing process comprises the following steps: (1) forming a fluorescent film support with five light-emitting faces through a mold, and forming a solid crystal cavity in the fluorescent film support; and (2) inversely placing an inverted LED chip into the solid crystal cavity of the dried fluorescent film bracket, and combining the LED chip with the fluorescent film bracket by using glue. According to the method, the fluorescent film support is formed by injection molding through a mold, and after the fluorescent film support is completely dried, reverse die bonding is carried out through glue, so that damage to an electrode can be avoided, and deformation caused by the fact that the fluorescent film support is not cured is also avoided; and in addition, the package of the CSP is effectively realized by combining injection molding, electrical gluing and die bonding processes, the process is simple, and the stability is good.
Owner:HONGLI ZHIHUI GRP CO LTD
Data link layer device and packet encapsulation method thereof
ActiveUS20220070120A1Efficient encapsulationEfficient encapsulating mechanismSpecial service provision for substationError prevention/detection by using return channelEmbedded systemLink layer
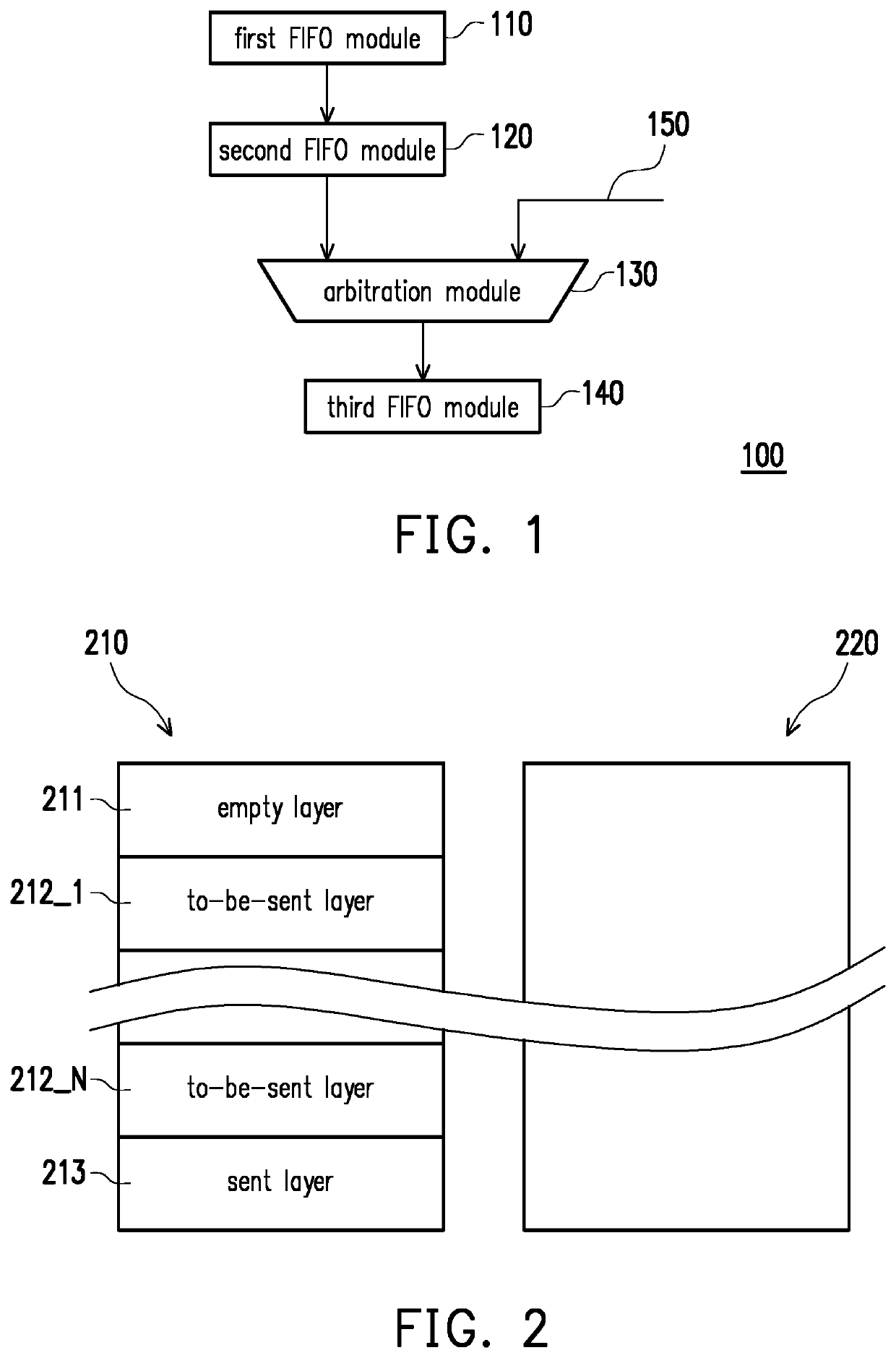
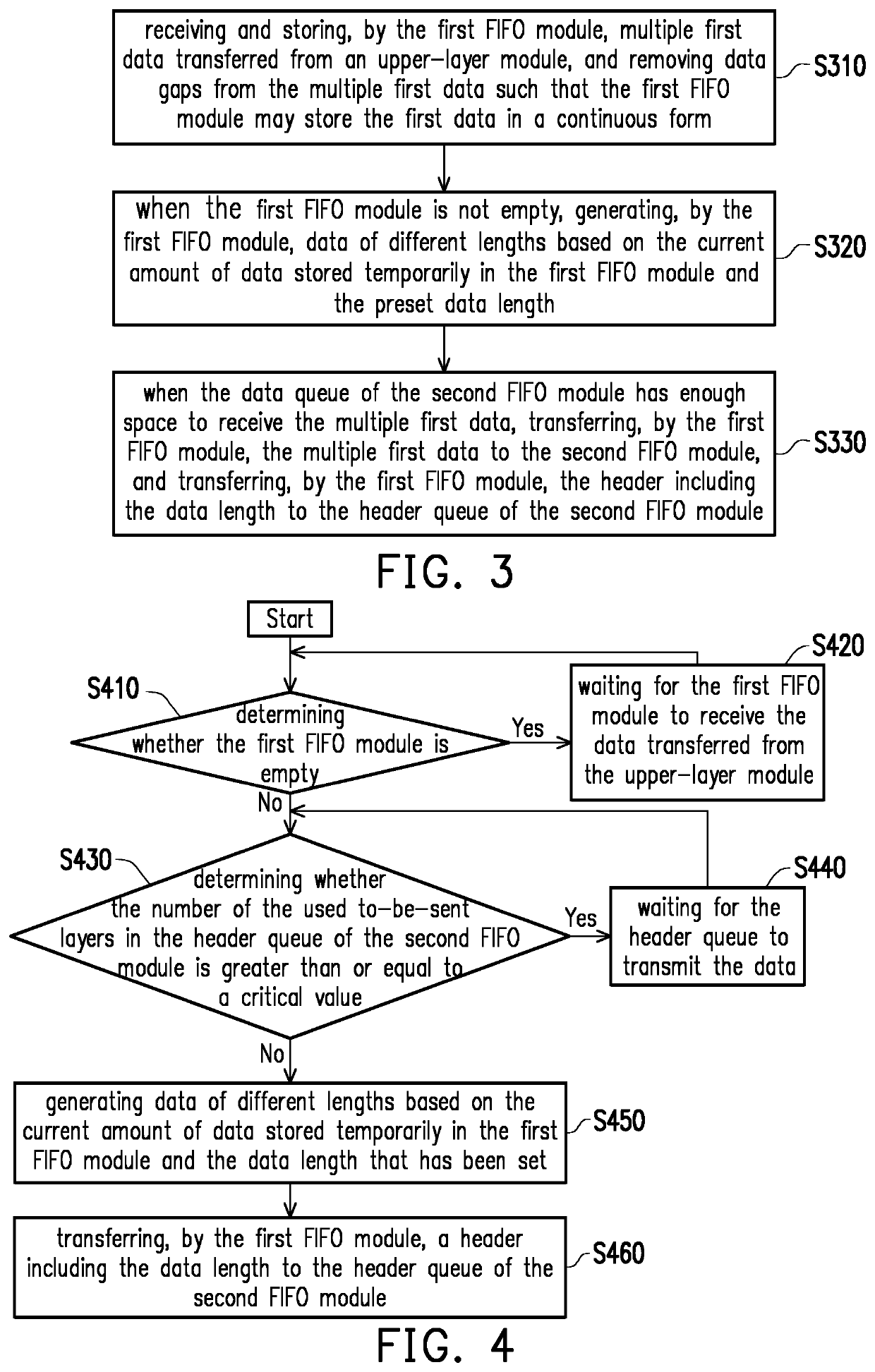
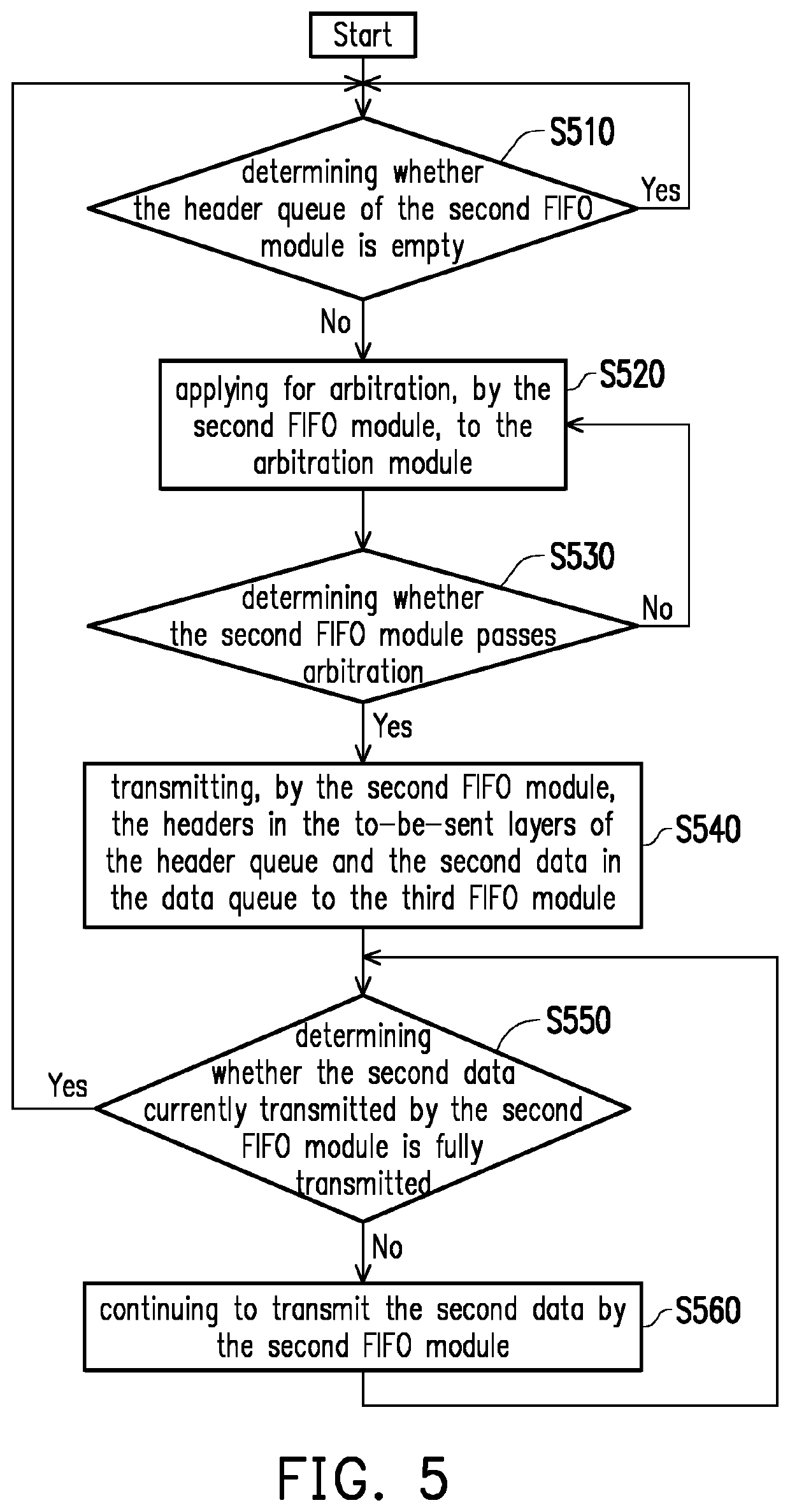
A data link layer device and a packet encapsulation method are provided. The data link layer device includes a first and a second first-in-first-out (FIFO) module. The first FIFO module receives and stores multiple first data from an upper-layer module, and removes data gaps from the first data to store the first data in a continuous form. When the first FIFO module is not empty, the first FIFO module generates data of different lengths based on the current amount of data stored temporarily in the first FIFO module and a preset data length. When the data queue of the second FIFO module has enough space to receive the first data, the first FIFO module transfers the first data to the second FIFO module, and the first FIFO module transfers a header including the data length to a header queue of the second FIFO module.
Owner:VIA ALLIANCE SEMICON CO LTD
Modified succinyl chitosan, drug-loaded nanoparticles and their application in the preparation of drugs targeting liver cancer cells
ActiveCN114316086BHydrophilicHydrophobicNanomedicinePharmaceutical delivery mechanismDrug targetPharmacology
The present application discloses modified succinyl chitosan, drug-loaded nanoparticles and their application in preparing drugs targeting liver cancer cells. The modified succinyl chitosan has the molecular structure shown in formulas II to XI. By introducing a long-chain alkyl group and an amino acid with dihydroxyl groups at the same time, the prepared modified succinyl chitosan has both hydrophilic and hydrophobic properties, thereby showing efficient encapsulation of sorafenib, And it is beneficial to its slow release and thermal stability. In addition, this application also confirmed the targeting of the drug-loaded nanoparticles prepared from this type of modified succinyl chitosan to liver cancer cells through in vitro cell experiments and in vivo experiments in liver cancer tumor-bearing mice, and can effectively reduce tumor-bearing The tumor volume of mouse liver plays a real anti-tumor effect.
Owner:GUANGXI MEDICAL UNIVERSITY
Nano realgar compound medicine and preparation method and application thereof
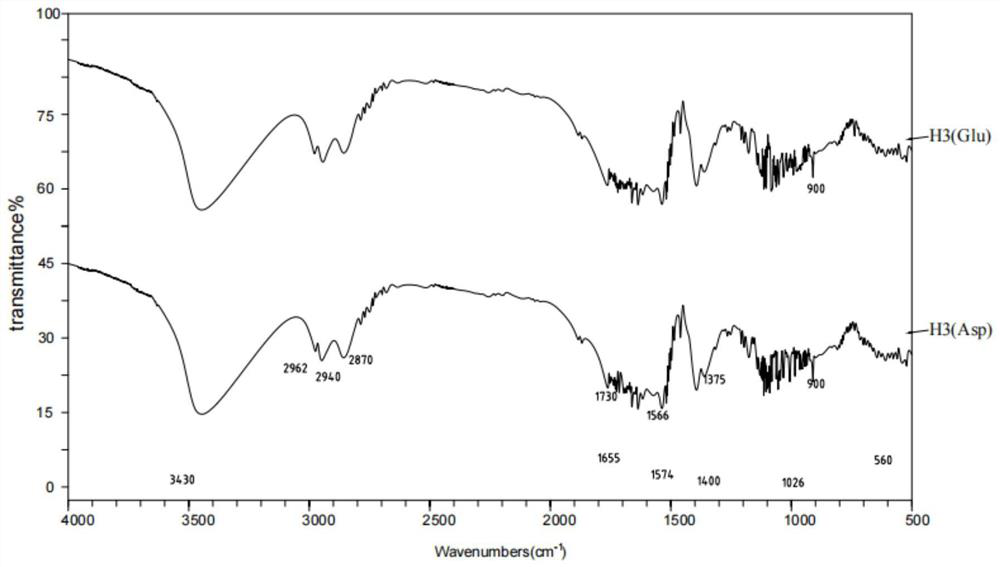
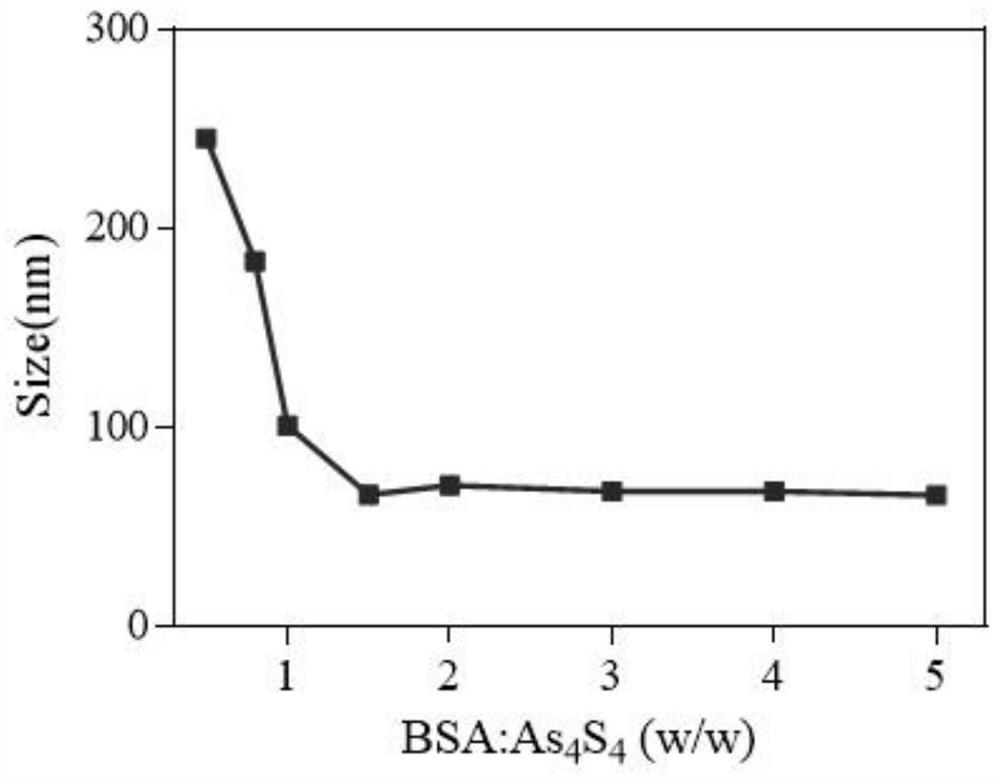
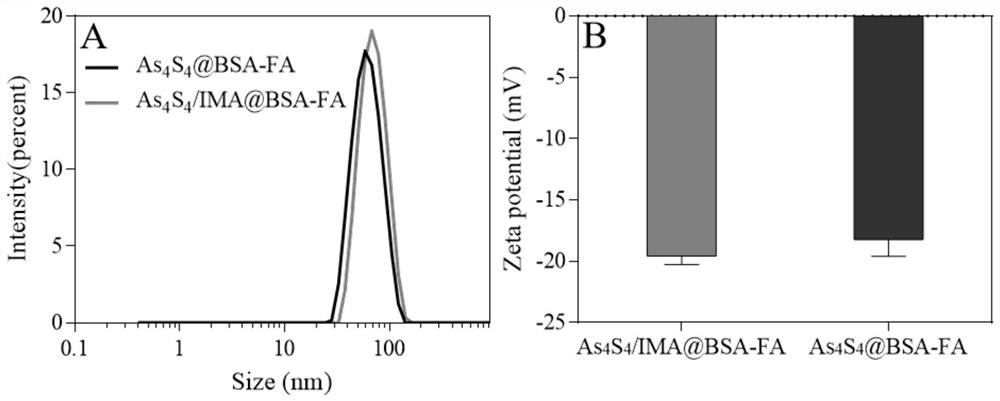
ActiveCN111840254BEfficient encapsulationProlong the residence time in the bodyOrganic active ingredientsInorganic active ingredientsMitochondrial pathwayApoptosis
The invention provides a nano-realgar compound medicine and a preparation method and application thereof. The nano-realgar compound medicine mainly comprises As 4 S 4 , BSA, IMA and FA. Its preparation method is as 4 S 4 ‑Ethylenediamine molecular cluster solution is the precursor, and the acid-base reaction is used to make As 4 S 4 Nucleate growth under the stabilization of BSA to form nanoparticles. At the same time, IMA was loaded into the hydrophobic domain of BSA by utilizing the hydrophobic force. The nanocomposite drug can be further modified with targeting ligands to increase the targeting and therapeutic index of tumor cells. The nano-realgar composite drug of the present invention can down-regulate the BCR-ABL protein level, induce apoptosis of chronic myeloid leukemia cells from the mitochondrial pathway, and deliver the two drugs to the treatment site in a specific ratio, so as to maximize the curative effect and reduce potential toxic and side effects.
Owner:HUNAN PROVINCIAL TUMOR HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com