Apelin liposome and preparation method thereof
A liposome and phosphatidylglycerol technology, applied in liposome delivery, pharmaceutical formulations, peptide/protein components, etc., can solve the problems of central nervous system toxicity, teratogenicity, and difficulty in large-scale production, and achieve simple preparation methods , long half-life and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] prescription:
[0042] [Pyrl]-Apelin-13
0.058mmol
DSPG
0.116mmol
DSPC
0.116mmol
CH
0.116mmol
DSPE-mPEG2000
0.012mmol
10g
5.5g
Sterile water for injection
Add to 100mL
[0043] Process:
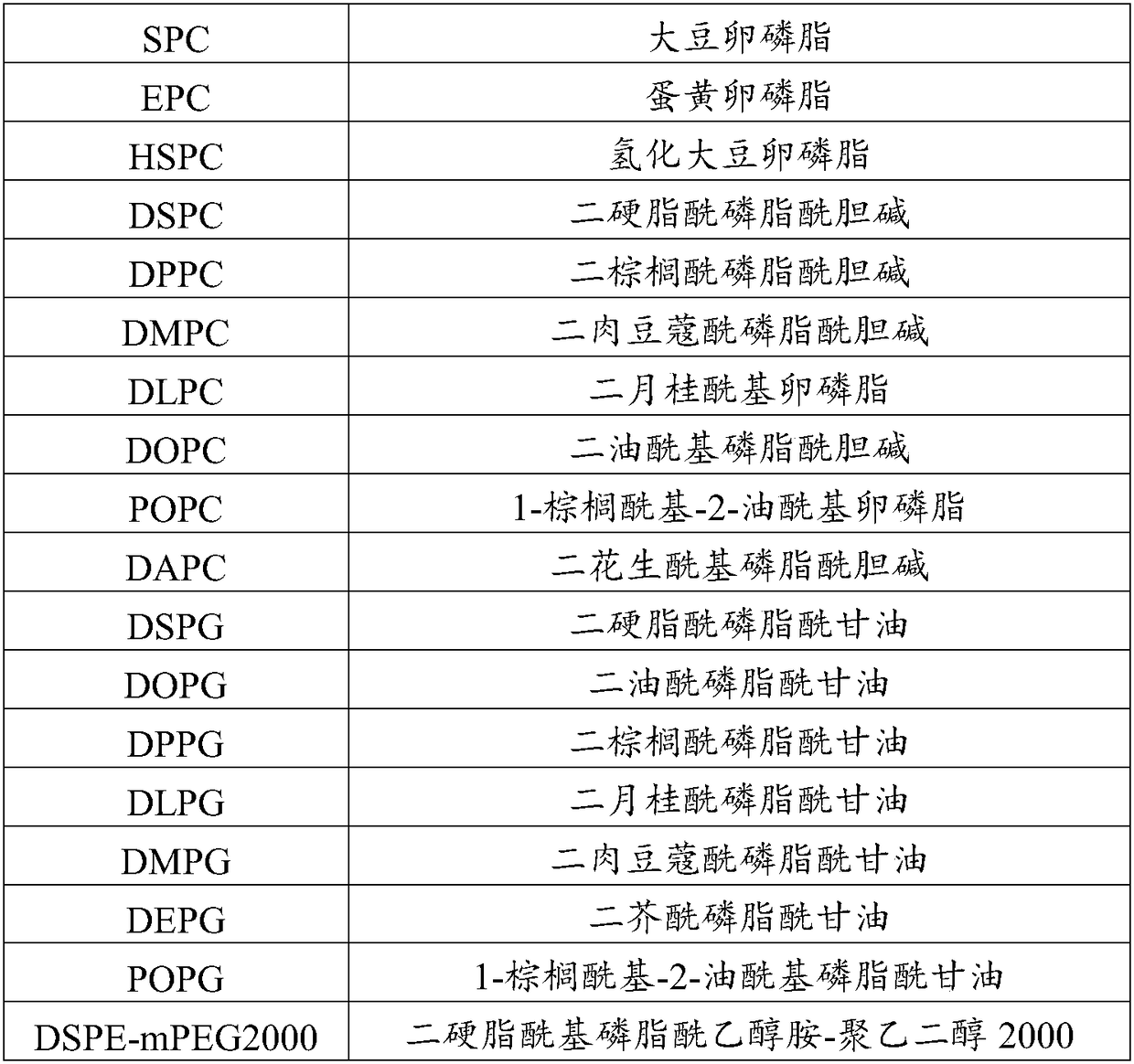
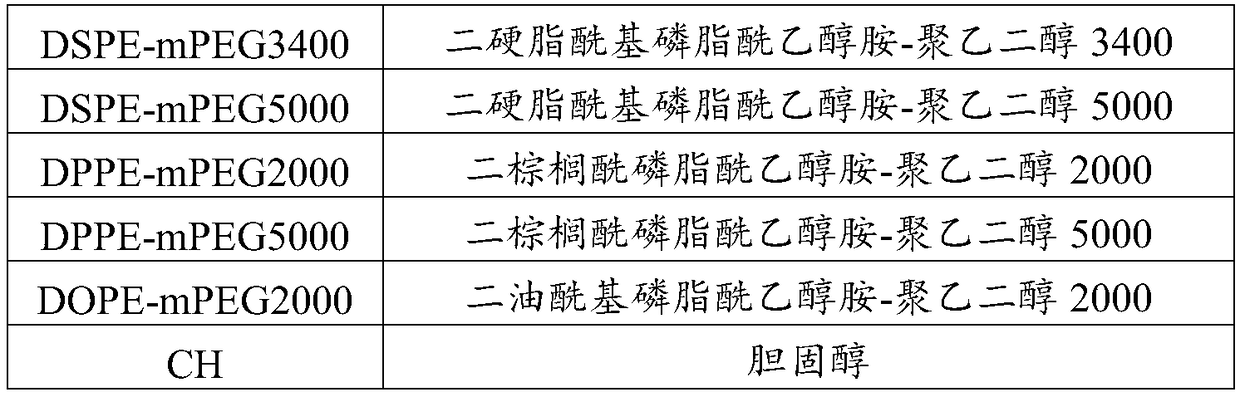
[0044] (1) Preparation of the organic phase: Apelin, phosphatidylcholine, phosphatidylglycerol, cholesterol, and phosphatidylethanolamine-polyethylene glycol derivatives are weighed in a container, and an appropriate amount of organic solvent is added to dissolve it completely , the system presents a homogeneous solution, and the organic solvent is selected from ethanol or ethanol / water mixed solution;
[0045] (2) Preparation of the water phase: Weigh the lyoprotectant in the above prescription amount, and dissolve it completely with an appropriate amount of sterilized water for injection under the condition of a water bath at 50° C. to 75° C. to obtain the water phase;
...
Embodiment 2~45
[0052] Examples 2-45 were prepared according to the prescriptions shown in the following Tables 3-5, using the same preparation method as in Example 1 to obtain Apelin liposomes. The encapsulation efficiency was measured by the same measuring method as in Example 1, and the results are shown in Tables 3-5 together.
[0053]
[0054]
[0055]
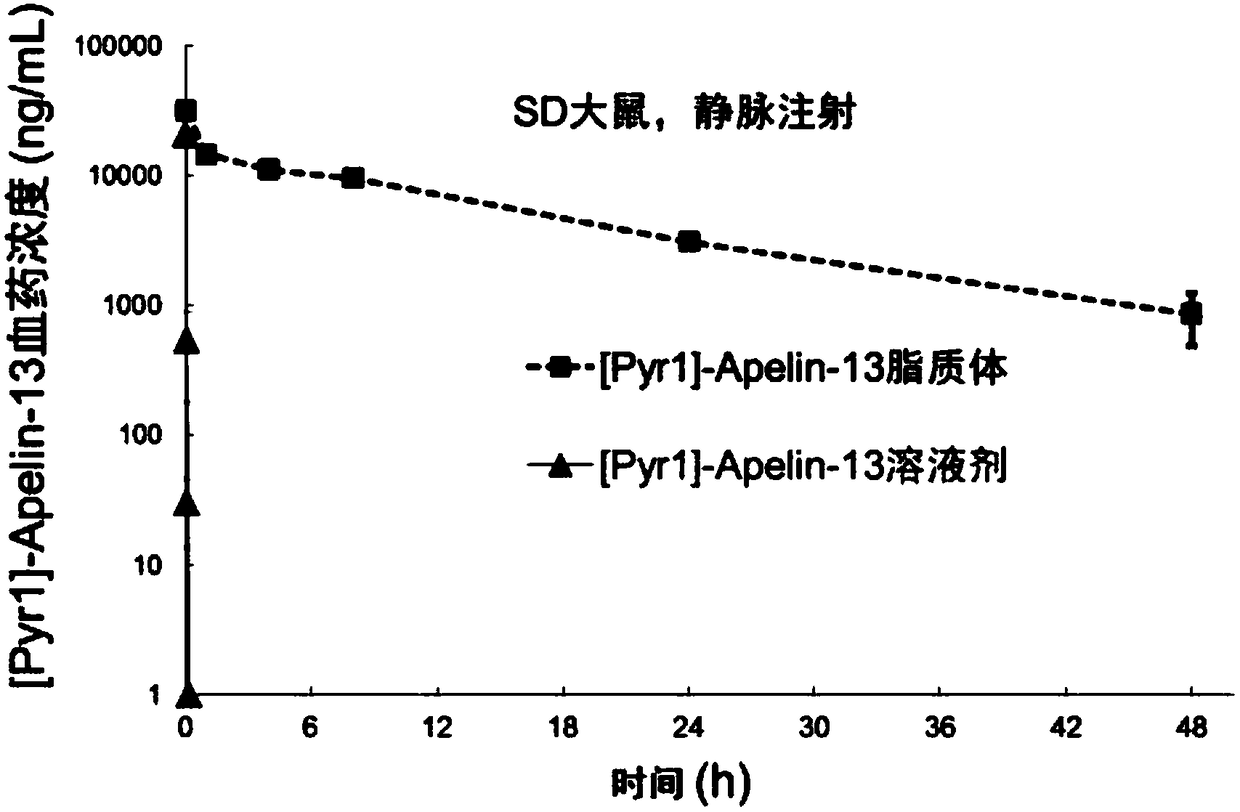
[0056] From the above-mentioned Examples 1-45, it can be known that the present invention can obtain Apelin liposomes with an encapsulation rate as high as 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com