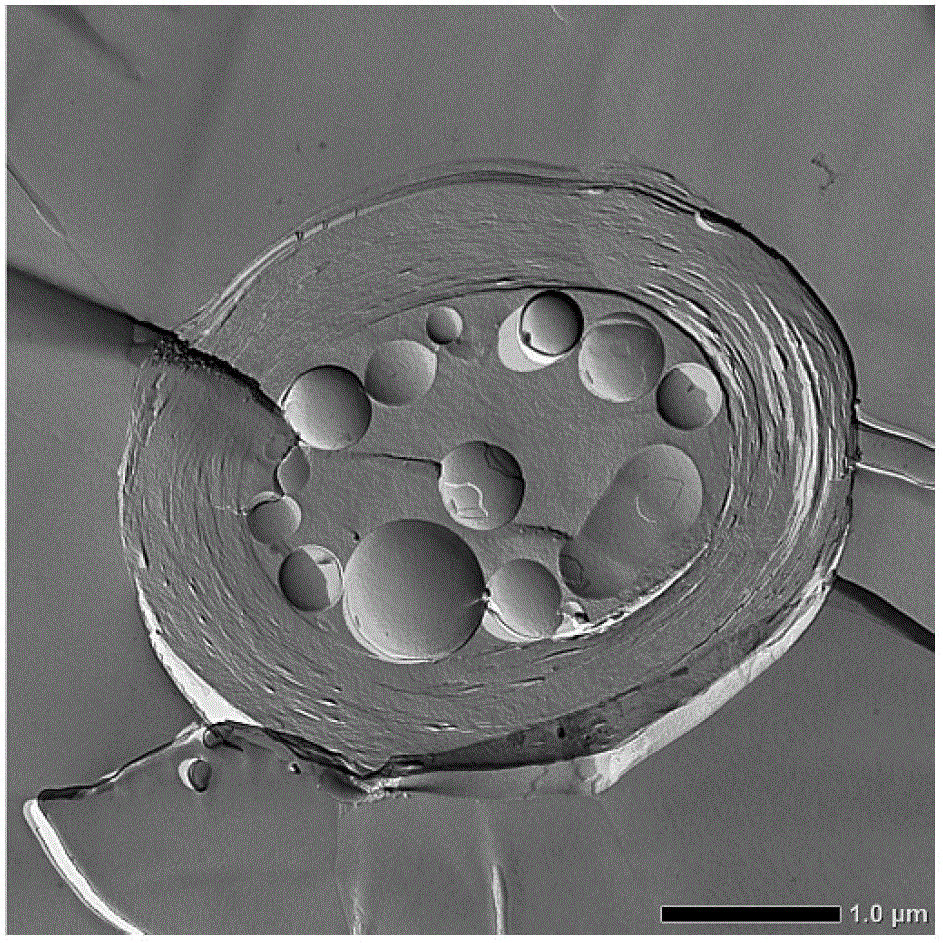
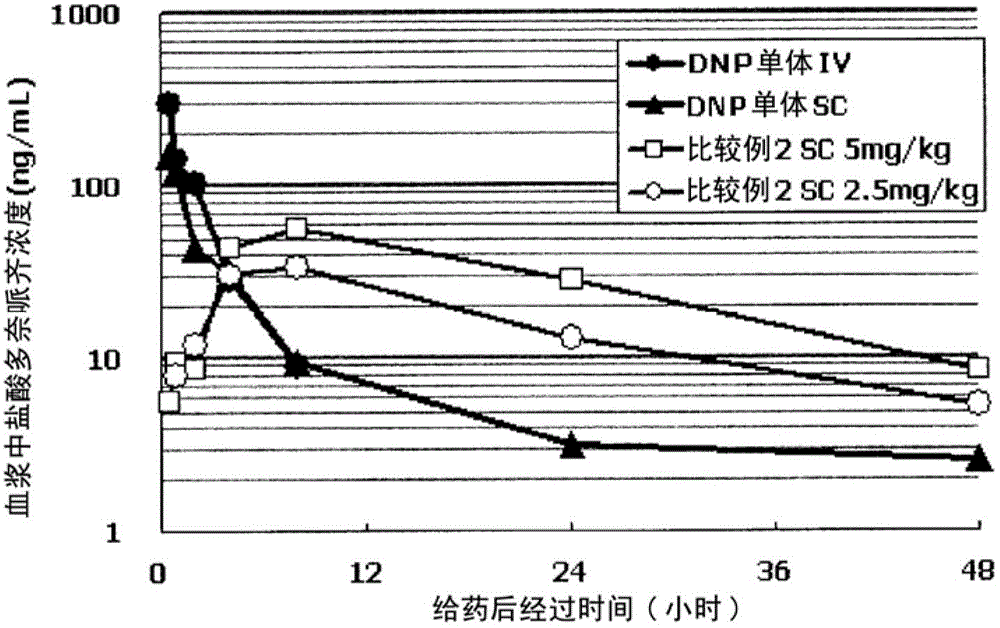
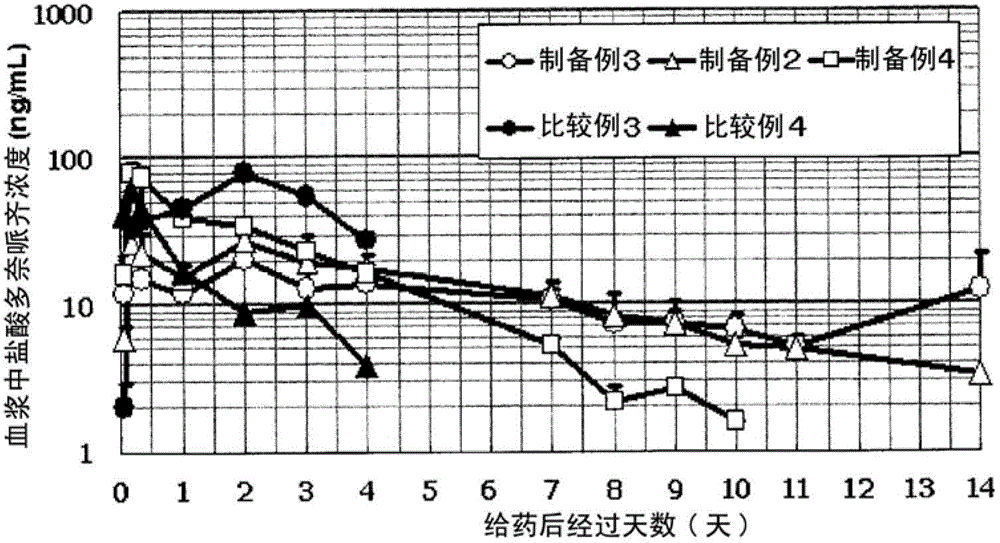
Liposome composition and its production method
A technology of a liposome composition and a manufacturing method, which is applied in the directions of liposome delivery, drug delivery, pharmaceutical formulation, etc., can solve the problems of drug encapsulation amount and slow-release time that cannot be said to be sufficient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] In the preparation of the liposome composition of the present invention, the water-miscible solvent comprising lipid and the first internal aqueous phase solution added therein can be set as 0.7 to 2.5, preferably 1.0 to 2.0.
[0072]
[0073] In the present invention, after adding the first internal aqueous phase solution to the water-miscible solvent containing lipids to prepare the first emulsion, and then adding the second internal aqueous phase solution to the first emulsion, the second internal aqueous phase solution The aqueous phase solution is not particularly limited. Examples thereof include the same solution as the first inner water phase, HEPES solution, NaCl solution, and aqueous solutions of sugars such as glucose or sucrose, and the same solution as the first inner water phase is preferable. In addition, both the first internal water phase and the second internal water phase are most preferably aqueous ammonium sulfate. The volume ratio of the first ...
Embodiment
[0085] Next, although an Example is given and this invention is demonstrated in more detail, this invention is not limited to these Examples.
[0086] The respective concentrations and particle diameters of the drug-encapsulated liposomes prepared in each example were determined according to the following methods.
[0087] Phospholipid concentration (mg / mL): The phospholipid concentration in the liposome suspension was quantified using high performance liquid chromatography or phospholipid quantification.
[0088] Cholesterol concentration (mg / mL): Cholesterol concentration in liposome suspension quantified using high performance liquid chromatography.
[0089] Total lipid concentration (mol / L): total molar concentration (mM) of lipids as membrane constituents calculated from the above-mentioned phospholipid concentration and cholesterol concentration.
[0090] Drug concentration (mg / mL): the liposome composition obtained above is diluted with RO water (reverse osmosis membra...
preparation example 1~4
[0103] (1) Preparation of empty liposomes
[0104] In order to make HSPC / Chol = 54 / 46 (molar ratio; hereinafter the same), 1.41 g of HSPC and 0.59 g of cholesterol were weighed, respectively, and 4 mL of absolute ethanol was added and heated to dissolve. Add 100mM (preparation example 1), 150mM (preparation example 2), 250mM (preparation example 3) ammonium sulfate aqueous solution or 300mM citric acid aqueous solution (pH3.0) (preparation example 4), heated and stirred for about 10 minutes to form an emulsion. Further, 10 mL of 20 mM HEPES / 0.9% sodium chloride (pH 7.5) heated to about 70° C. was added to this emulsion, followed by heating and stirring for about 10 minutes. The liposomes after the warming was completed were quickly cooled with ice.
[0105] (2) Formation of pH gradient
[0106] Add 20mM HEPES / 0.9% sodium chloride (pH 7.5) to the liposomes obtained above and disperse, centrifuge at 3500rpm for 15 minutes to precipitate the liposomes. Thereafter, the superna...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com