Pharmaceutical compositions containing taxane-cyclodextrin complexes, method of making and methods of use
A cyclodextrin and composition technology, which is applied in the field of taxane compound pharmaceutical preparations, can solve the problems of increased risk of cytotoxic compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
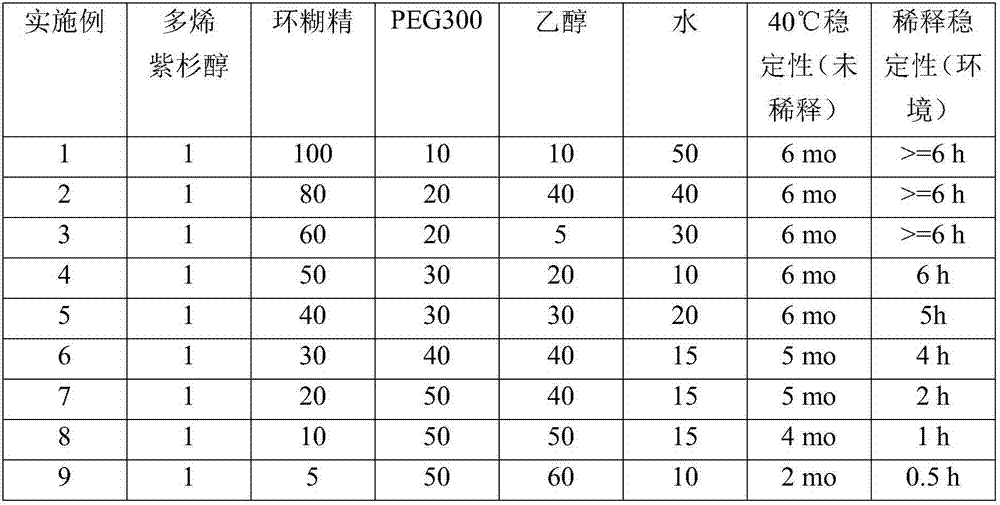
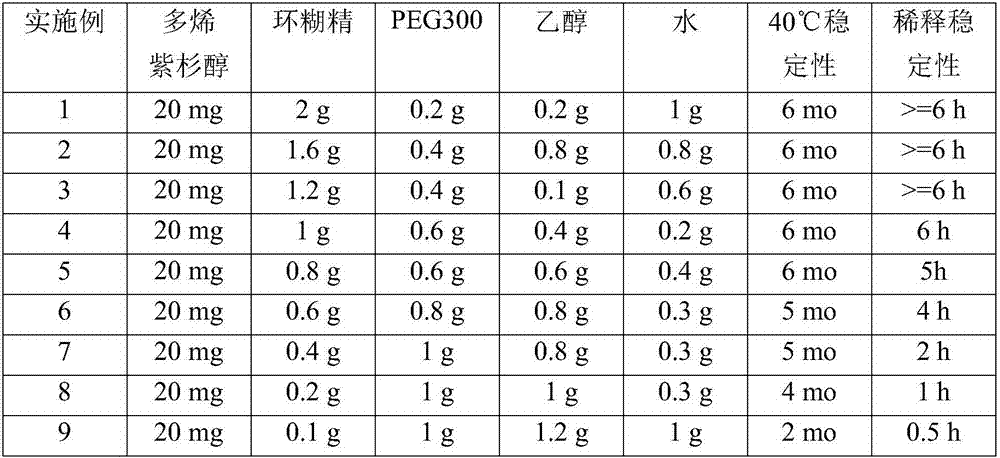
Examples
Embodiment
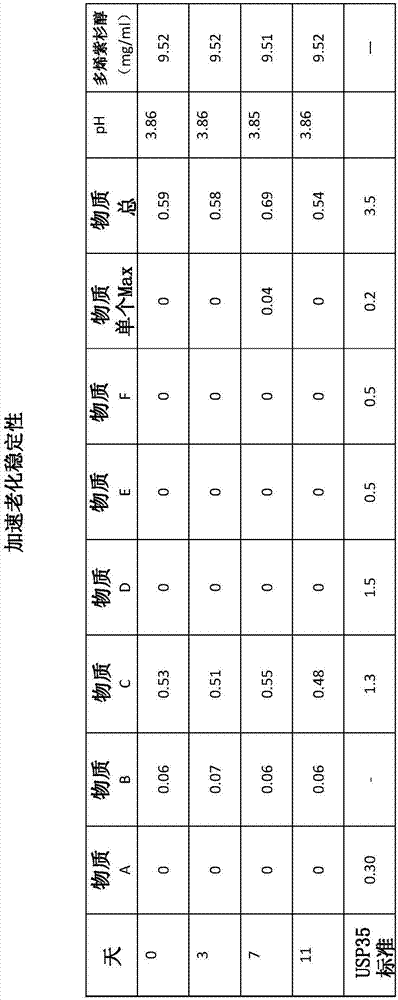
[0069] Accelerated Aging Stability Study
[0070] The above liquid pharmaceutical composition comprising -10 mg / mL docetaxel was diluted and subjected to accelerated aging at 40°C for 11 days. Impurity levels specified by USP35 (United States Pharmacopeia 35) for taxane compositions were analyzed by HPLC at timed intervals. Stability was tested on day 0 at 6 hours. The impurity levels in the liquid pharmaceutical compositions were compared to the tolerable levels according to the USP35 standard. The results are shown in figure 1 middle. It can be seen that all impurities (Substances A-F) are well below the USP35 limit for the full 1 day period of accelerated aging. Furthermore, during the 11 days tested, there was no increase in the impurities present and no new impurities or degradation products were formed, indicating that the composition is chemically stable. The composition is also physically stable as no precipitate or haze is formed.
[0071] Furthermore, the con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com