Use of anti cd70 antibody argx-110 to treat acute myeloid leukaemia
A myeloid leukemia and antibody technology, applied in the direction of antibody medical components, antibodies, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problem that patients are not suitable for intensive chemotherapy and the burden of chemotherapy patients, etc. problem, to achieve the effect of reducing blast cells in AML
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0090]As demonstrated herein, patients with myeloid neoplasms, such as AML and MDS, can be treated with anti-CD70 antibodies. After a single administration of the anti-CD70 antibody, the number of leukemia stem cells capable of being isolated from the subject's bone marrow was significantly reduced, as was the number of blasts detected in the bone marrow and peripheral blood. This result was observed even at surprisingly low antibody doses.
[0091] Accordingly, in a first aspect, the present invention provides a method for treating acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS) in a subject, said method comprising administering to said subject One or more doses of the anti-CD70 antibody or antigen-binding fragment thereof are administered. The present invention provides a method for treating acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS) in a subject, said method comprising administering to said subject one or more therapeutically effe...
Embodiment 1
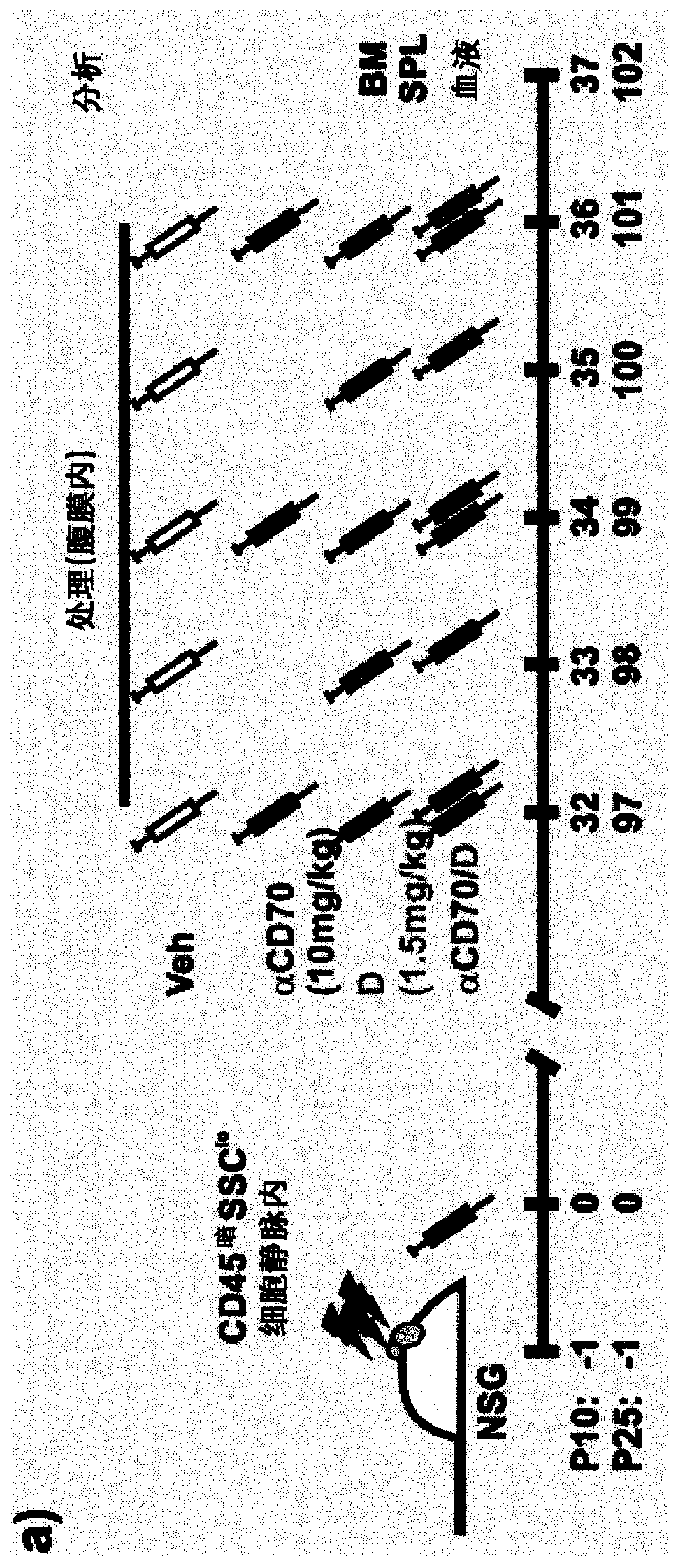
[0207] Example 1: Anti-CD70 Antibody Monotherapy or Combination with Decitabine on Human AML Transplanted in Mice The role of LSC
[0208] Transplant 5 × 10 into NSG mice 6 CD45 暗 SSC lo Human AML cells. At 32 days post-implantation (implantation in PB: 14.45% + / - 0.95%), NSG mice were randomized to receive vehicle (Veh), aCD70 mAb (aCD70, ARGX-110, 10 mg / kg), ground Citabine (D, 1.5 mg / kg / day) or combination (aCD70 / D) treatment was continued for 5 days and bone marrow, spleen and blood were analyzed.
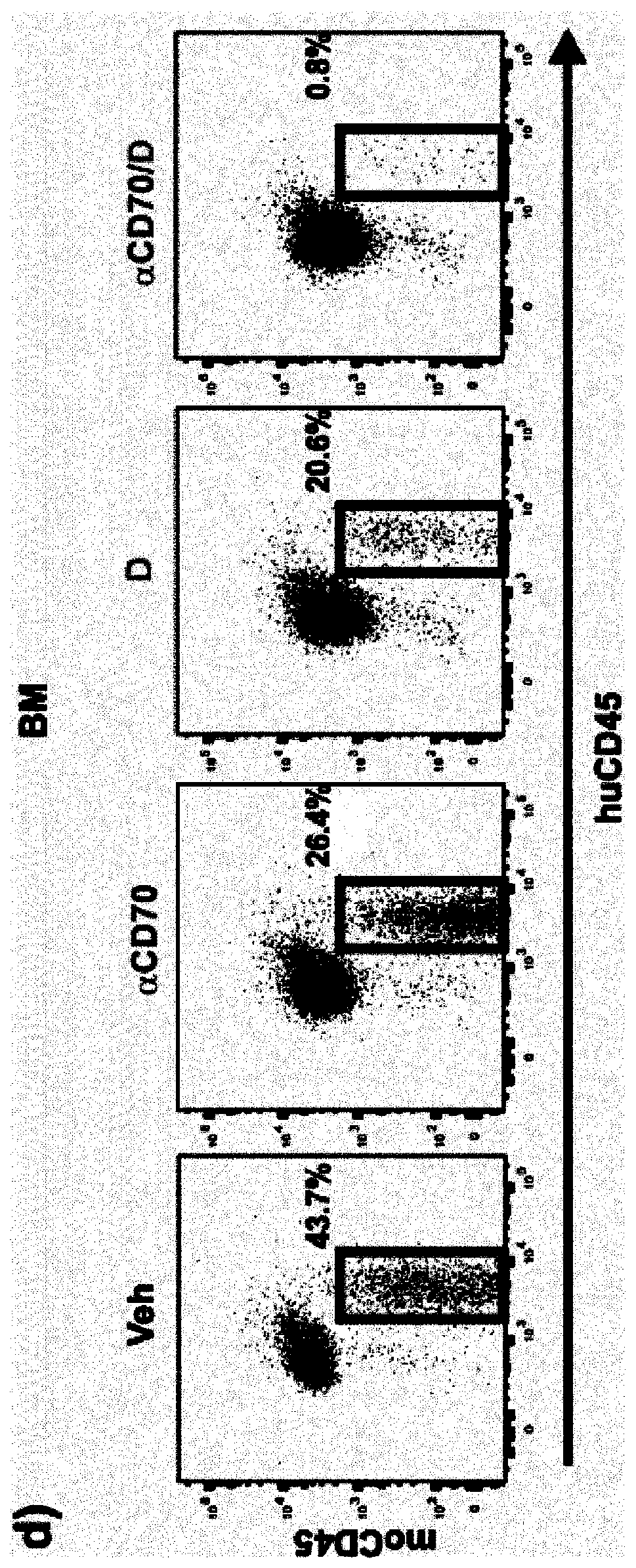
[0209] Both anti-CD70 and decitabine alone caused decreased total engraftment in bone marrow, spleen and blood ( figure 1 ). The combination of anti-CD70 and decitabine enhanced the reduction in the percentage of engrafted human cells compared to either therapy alone ( figure 1 ).
[0210] The combination therapy also reduced CD34+ AML cells (a marker of progenitor cells) in the bone marrow better than decitabine or anti-CD70 alone ( figure 1 ). Furthermore, anti-C...
Embodiment 2
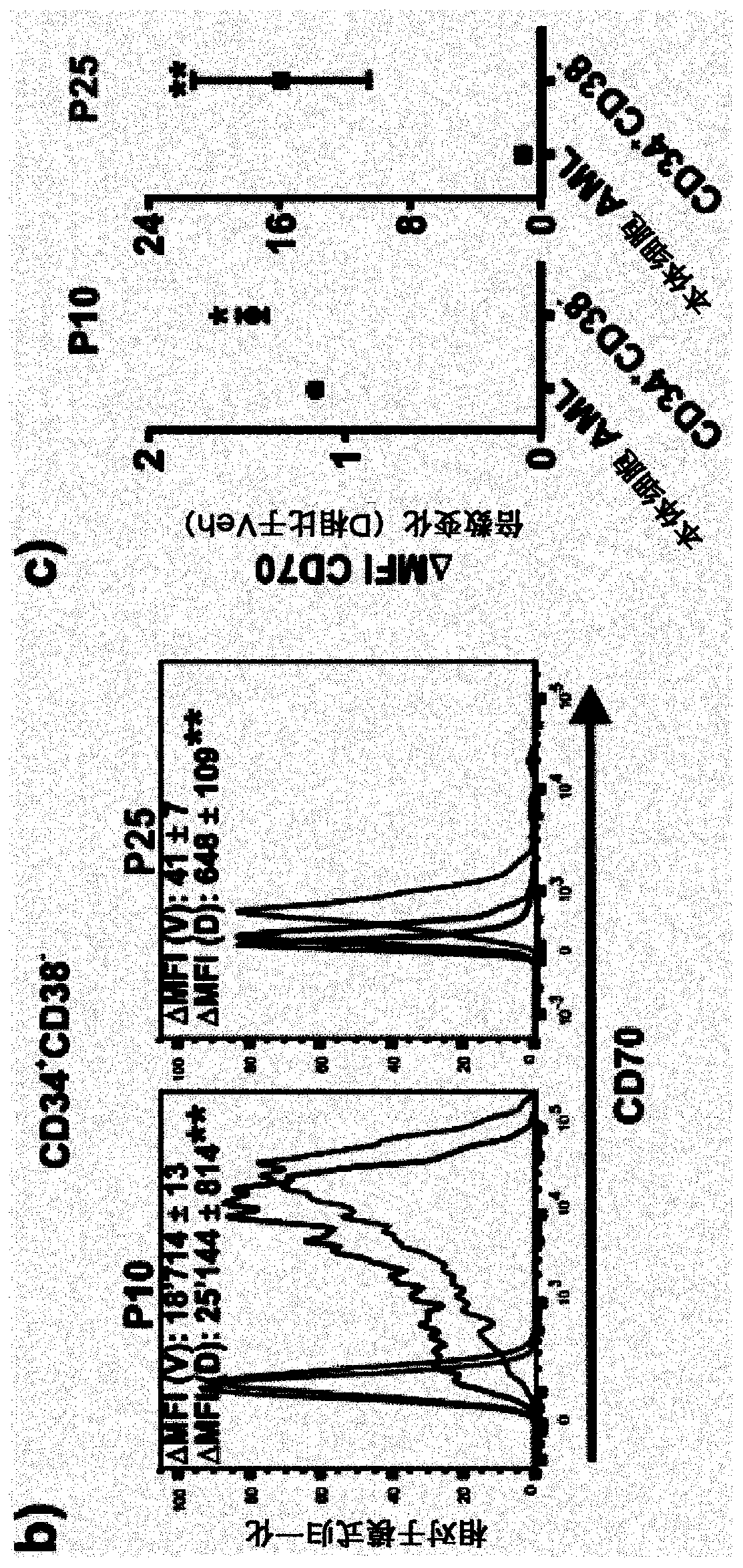
[0211] Example 2: Hypomethylating agent (HMA) upregulates CD70 expression on primary AML stem cells in vitro and in vivo, which is consistent with The combination of anti-CD70 antibody and HMA enhances the reduction of AML colony formation
[0212] Further studies in primary human AML LSCs found that anti-CD70 antibody treatment in combination with nucleoside metabolism inhibitors (NMIs), such as the hypomethylating agent decitabine, enhanced AML blast engraftment in mice reduction.
[0213] The effect of NMI treatment (for example HMA such as azacitidine or decitabine) on CD70 expression of AML LSCs was investigated. CD34+CD38- cells were isolated from AML patients and cultured in the presence of 0.5 mM decitabine or vehicle.
[0214] figure 2 Data in A demonstrates increased CD70 expression of AML LSC cells when cells were cultured with decitabine. This increase in CD70 expression in response to decitabine occurred in cells obtained from AML patients in all disease r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com