Application of pharmaceutical composition in preparation of drug for treating acute myeloid leukemia (AML)
A composition and leukemia technology, applied in the fields of medicinal chemistry and cell biology, can solve the problems of high recurrence rate, low long-term disease-free survival rate, and no related reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
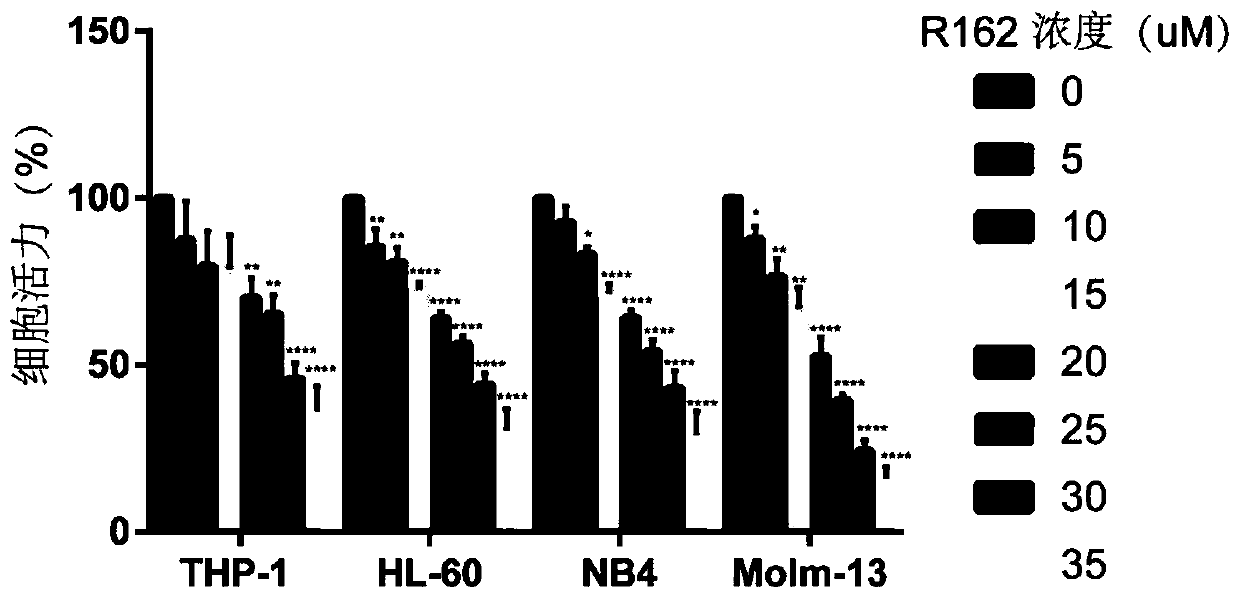
[0022] Example 1: Effect of R162 on proliferation of AML cell lines
[0023] Take the AML cell lines THP-1, HL-60, Molm-13, and NB4 in the logarithmic growth phase and count them by centrifugation, and count them at 1×10 5The cells / well density were planted in 6-well plates, the complete medium was used as a blank control, and R-162 was added to the experimental group to a final concentration of 5, 10, 15, 20, 25, 30, 35 μM. On the 7th day of cell seeding, the cells were blown evenly and transferred into 96-well plates, four wells in each group, 100 μl per well, and 10 μl of CCK8 solution was added, 37 ° C, 5% CO 2 After incubation for 2 h, the absorbance (OD) was measured at a wavelength of 450 nm with a microplate reader. The OD of the experimental group / OD of the control group was used as an index to evaluate the cell viability.
[0024] The results of research on the inhibition of R162 on AML cell activity showed that R162 could inhibit the activity of THP-1, HL-60, Molm...
Embodiment 2
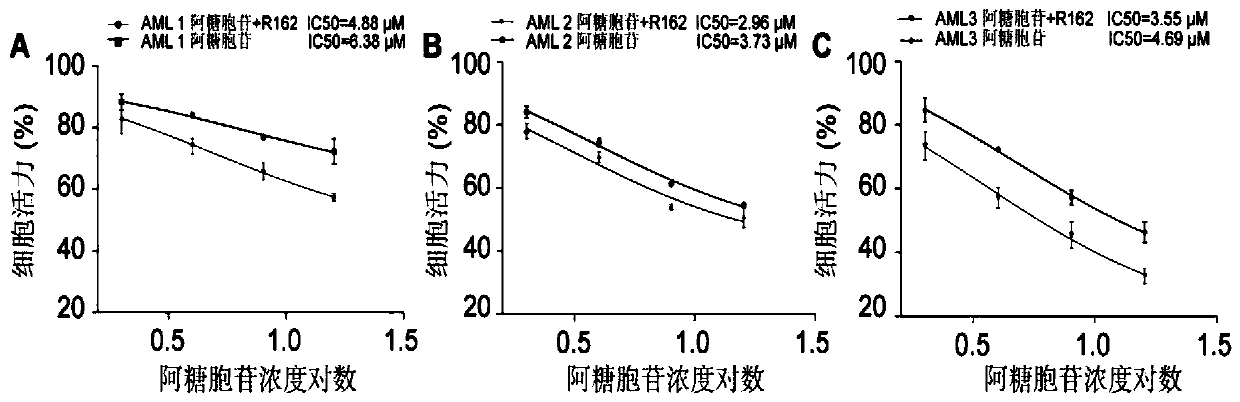
[0025] Example 2: In Vitro Study of Compositions for Treating AML
[0026] Obtain primary AML cells from newly diagnosed AML patients, add IMDM medium containing 10% fetal bovine serum, and culture at 37°C, 5% CO 2 cultured in an incubator. In the study of AML primary cells, the concentration of cytarabine in the composition was 0, 2, 4, 8, 16 μM, and the concentration of R162 was 20 μM. The experiment was divided into single use cytarabine group and cytarabine + R162 group . Place primary AML cells in a 6-well plate, 1×10 5 pcs / hole. After 48 hours of drug treatment, 100 μl of cells were inoculated into a 96-well plate, 10 μl of CCK-8 assay solution was added to each well, and incubated at 37°C for another 2 hours, the absorbance was measured by reading the plate at a wavelength of 450 nm, and each well was calculated. IC50 of group cells to cytarabine.
[0027] The results of primary cell experiments in 3 newly diagnosed AML patients showed that R162 can increase the se...
Embodiment 3
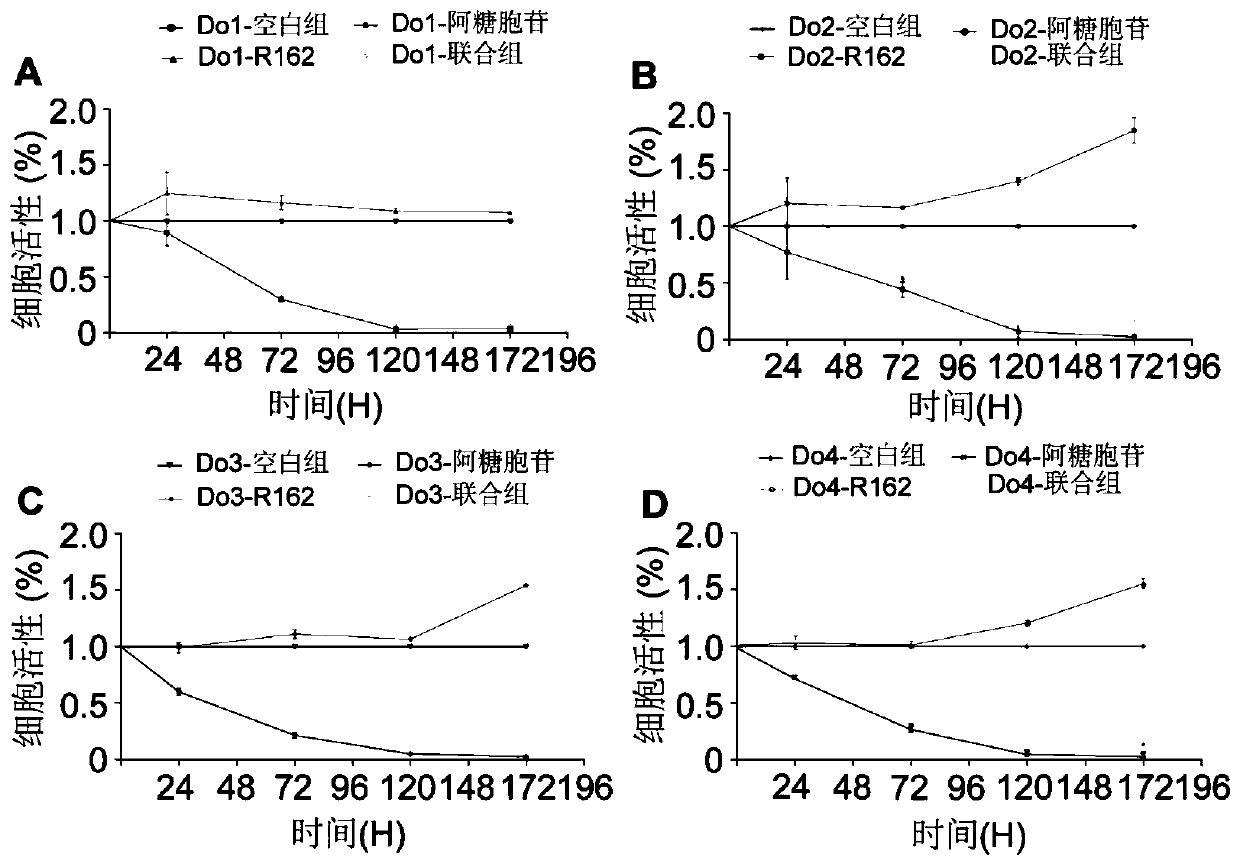
[0030] Example 3: In Vivo Study of Compositions for Treatment of AML
[0031] Female B-NSGTM (NOD-Prkdcscid II2rgtm1 / Bcgen) mice (6-8 weeks old) were used for AML modeling, and the tail vein was inoculated with 1×10 6 Molm-13-luc cells. D-luciferin (250 mg / kg) was injected intraperitoneally and imaged using the IVIS Lumina LT system. Before the treatment, the mice were randomly divided into four groups, namely the control group, the single cytarabine group, the single R162 group, and the combination group. Mice were observed and weighed daily, and leukemic burden was assessed every 7 days by bioluminescent imaging. Mice were treated with 20 mg / kg cytarabine, 20 mg / kg R162 in the composition. Cytarabine was diluted to 25 mg / mL in PBS and stored at -20°C. R162 was dissolved in DMSO to 100 mg / ml, and stored at -20°C in the dark. Cytarabine was administered daily by intravenous injection and R162 by intraperitoneal injection starting from day 8 after transplantation. In the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com