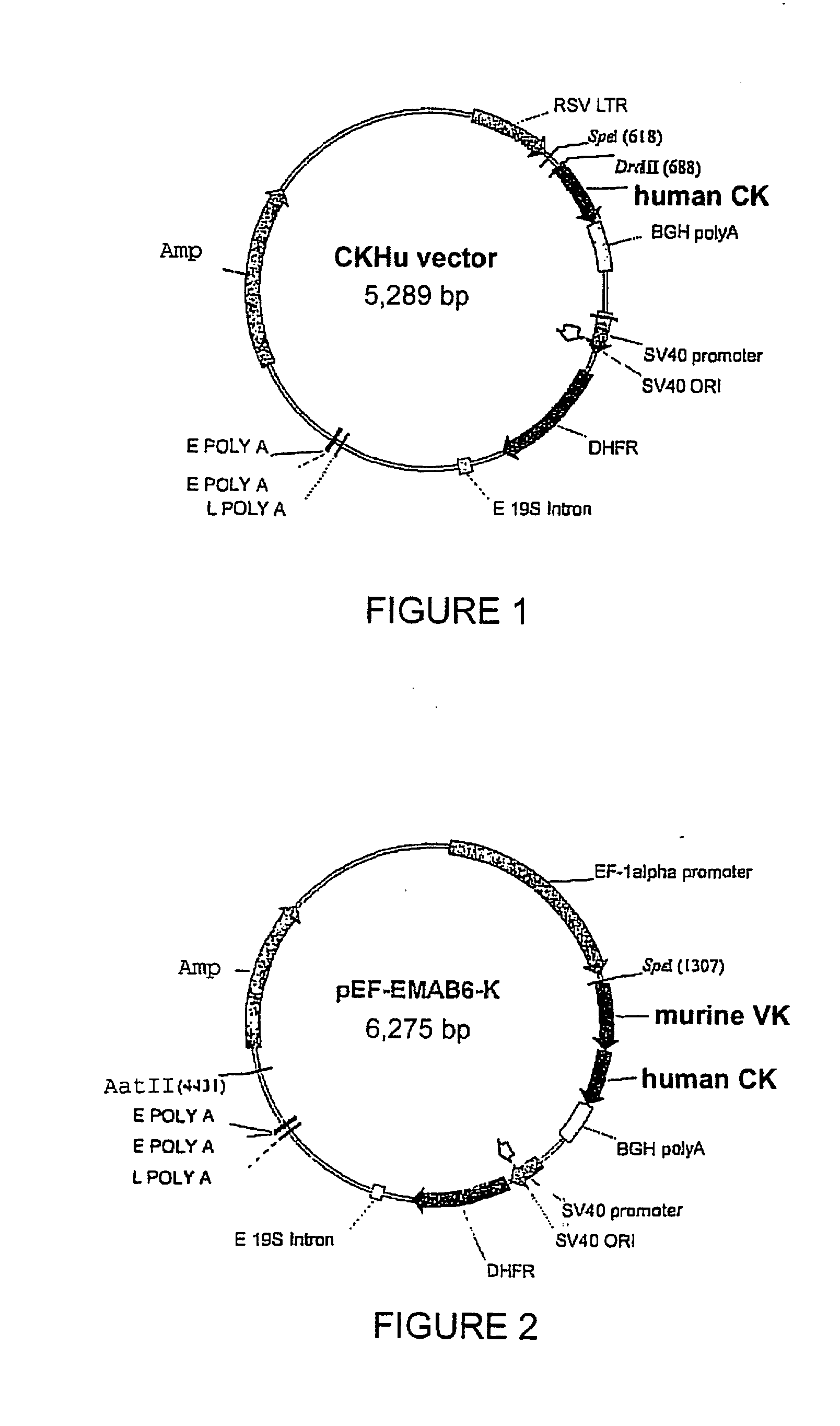
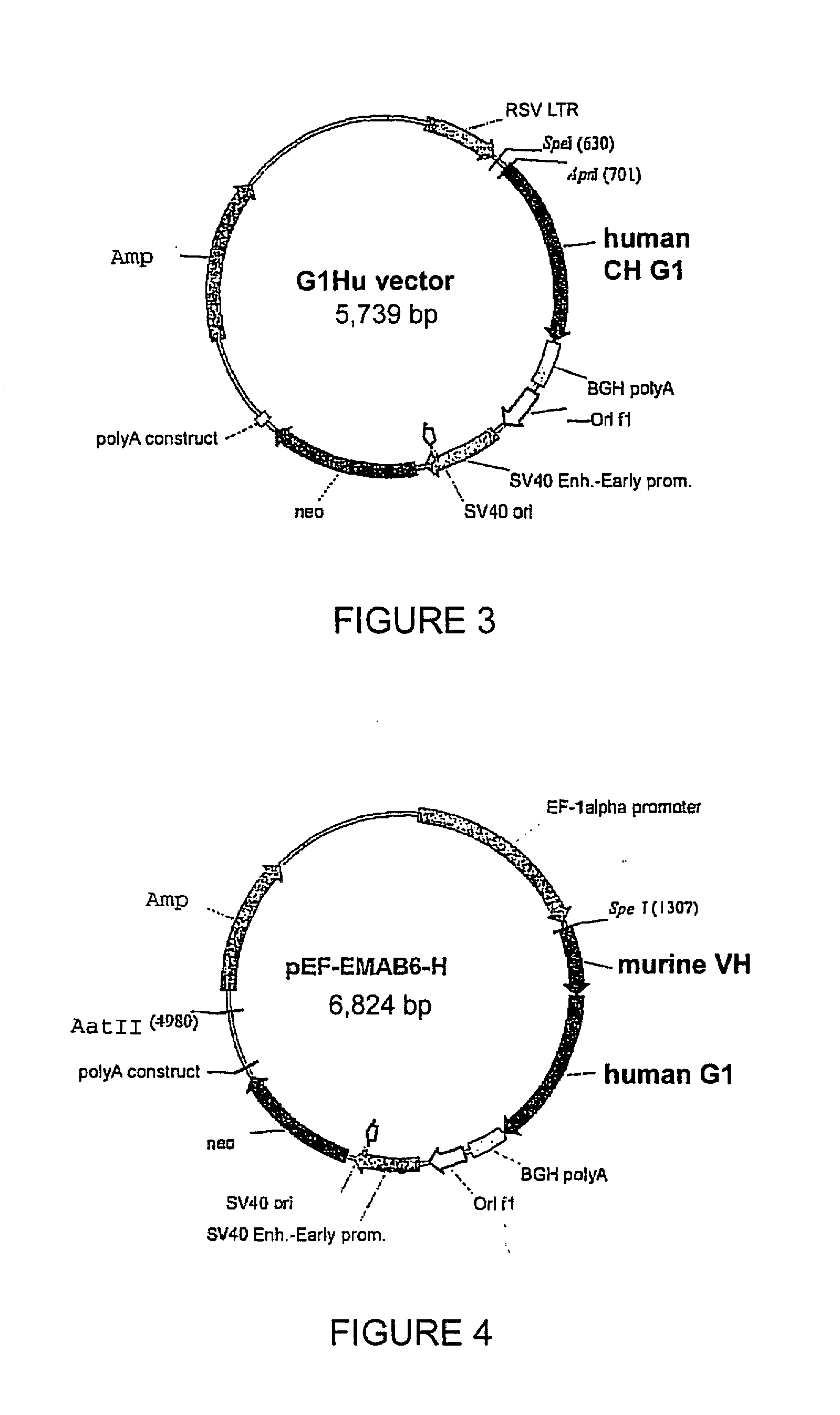
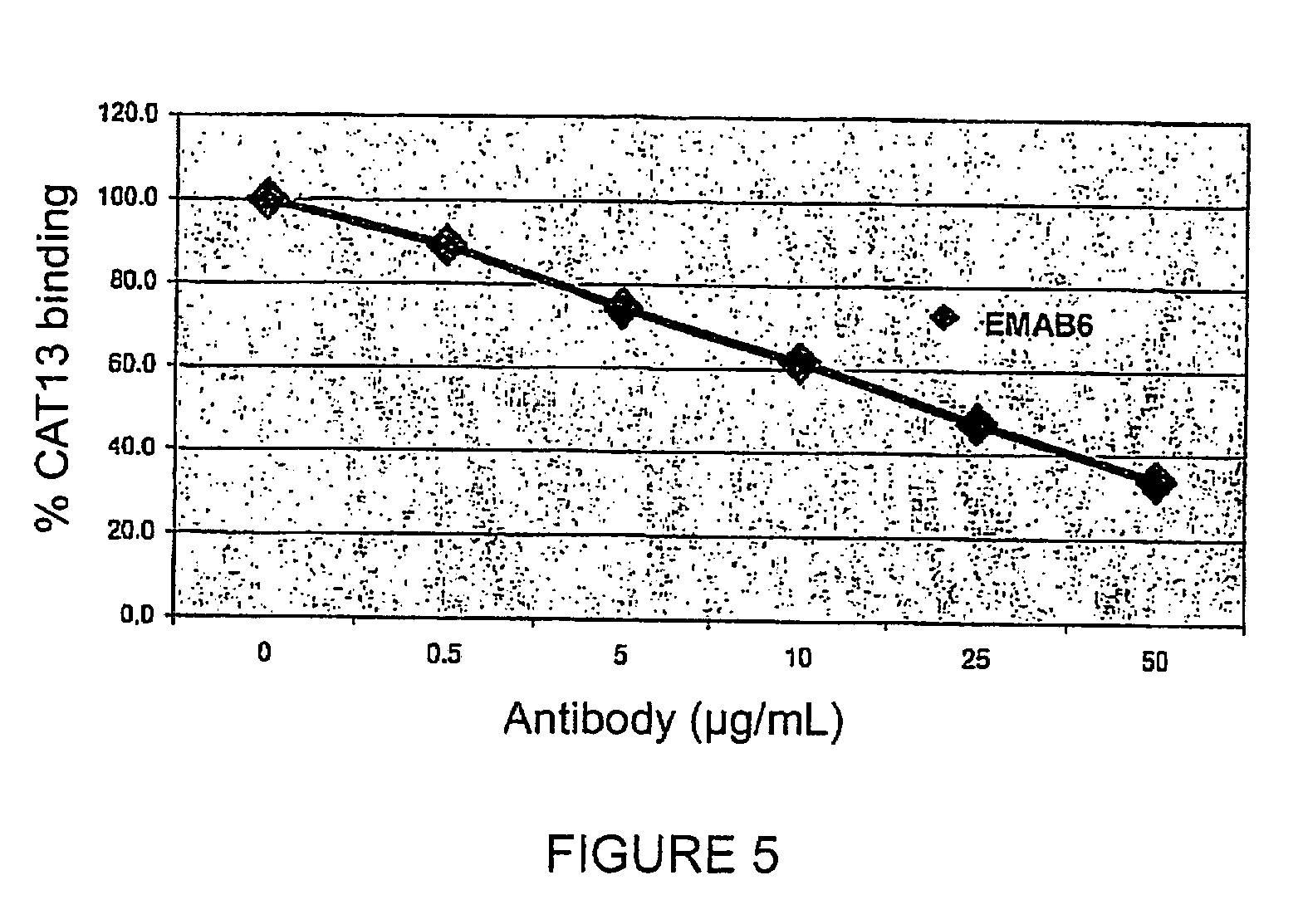
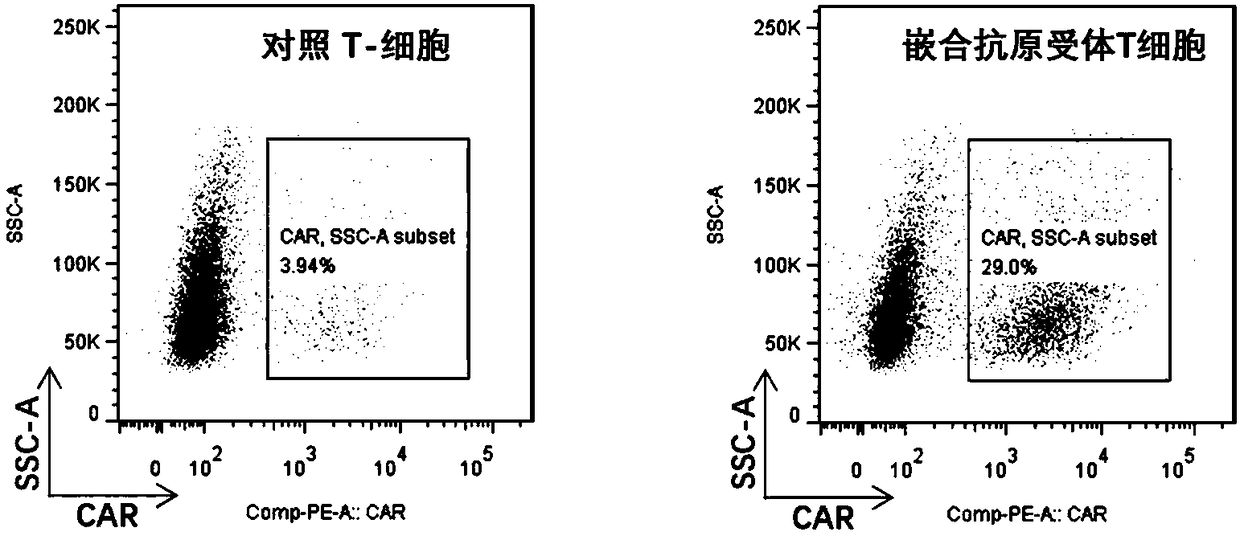
Patents
Literature
123 results about "Adult T-cell lymphoma/leukaemia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
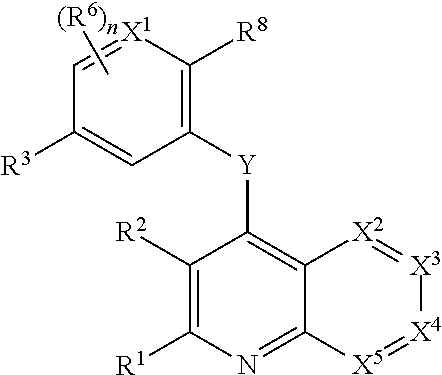
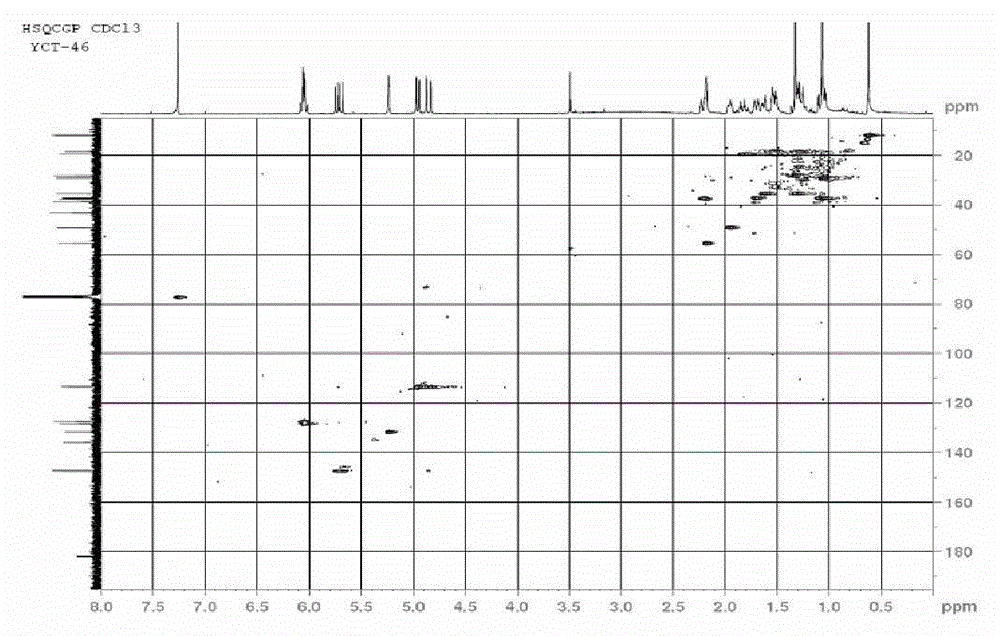
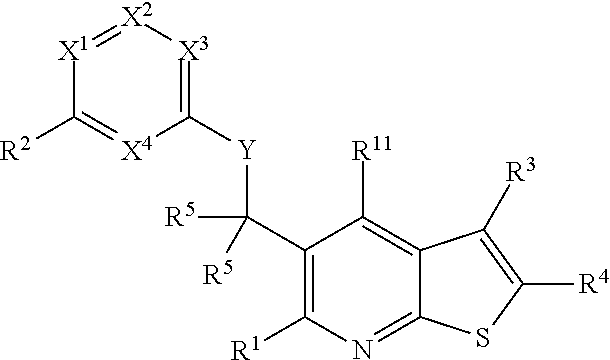
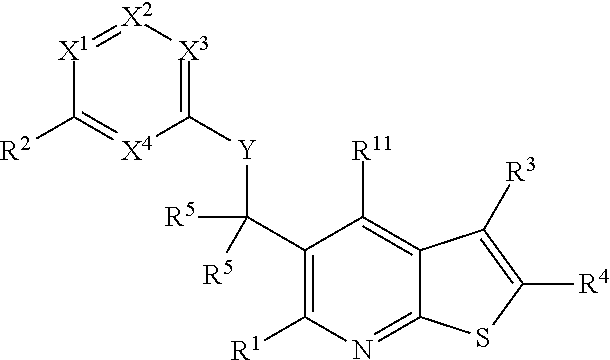
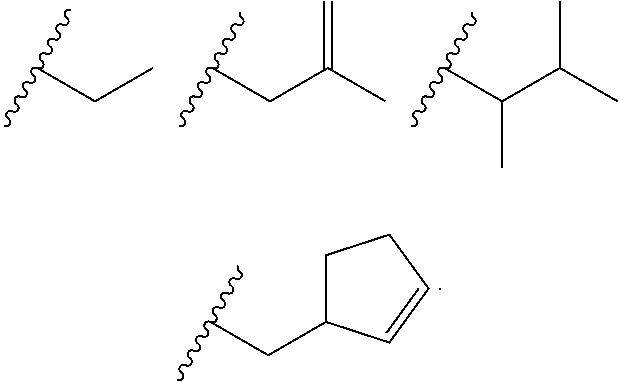
Heterocyclic compounds and their uses
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjogren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110δ activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
Compositions Comprising Human Embryonic Stem Cells and their Derivatives, Methods of Use, and Methods of Preparation
The present invention relates to a pharmaceutical composition comprising of preparations of human embryonic stem (hES) cells and their derivatives and methods for their transplantation into the human body, wherein transplantation results in the clinical reversal of symptoms, cure, stabilization or arrest of degeneration of a wide variety of presently incurable and terminal medical conditions, diseases and disorders. The invention further relates to novel processes of preparing novel stem cell lines which are free of animal products, feeder cells, growth factors, leukaemia inhibitory factor, supplementary mineral combinations, amino acid supplements, vitamin supplements, fibroblast growth factor, membrane associated steel factor, soluble steel factor and conditioned media. This invention further relates to the isolation, culture, maintenance, expansion, differentiation, storage, and preservation of such stem cells.
Owner:SHROFF GEETA
American cockroaches effective fraction for anti-tumor prepared by macroporous adsorption resin and use
ActiveCN101214262AAnthropod material medical ingredientsPharmaceutical delivery mechanismSolventChemistry
The present invention relates to a periplaneta americana extract effective part with cytotoxic activity, a preparation method and a medical purpose thereof. The periplaneta americana extract effective part of the present invention is refined from fresh or dry blattidae periplaneta americana body which is extracted by alcohol water, processed for macroporous resin column chromatography and is eluted by alcohol solvent. The periplaneta americana extract effective part obtained by the present invention has obvious cytotoxicity and internal anticancer efficacy towards oral epidermoid carcinoma (KB) cell, poorly differentiated gastric abenocarcinoma tumour (BGC-823) cell, human chronic myelogonium leukaemia cancer (K562) cell and human myelogonium leukaemia cancer cell (HL-60) and can be used for preparing for a drug and a health care product for remedying the tumours.
Owner:KUNMING SINOWAY NATURAL PHARMA
Serum-free medium for umbilical cord mesenchymal stem cells
InactiveCN105420182AOvercoming pollutionOvercoming Cell Expansion NumbersSkeletal/connective tissue cellsPancreatic hormoneStem cell culture
The invention provides a serum-free medium for umbilical cord mesenchymal stem cells and belongs to the technical field of cell culture. The serum-free medium for umbilical cord mesenchymal stem cells comprises a basal culture medium body and added ingredients, wherein the basal culture medium body is a DMEM culture medium; the added ingredients include a basic fibroblast growth factor hFGF, a epidermal growth factor hEGF, insulin hI, a leukaemia inhibitory factor hLIF and astragalus polysaccharide. The umbilical cord mesenchymal stem cells are subjected to subculture and amplification in the medium, and the surfaces of the cultured cells are marked and analyzed. The serum-free medium for umbilical cord mesenchymal stem cells overcomes the defect of exogenous pollution of serum and solves the problem of contradiction between the cell expansion number and cost reduction. The medium is free of serum, thereby preventing influence of animal-derived serum ingredients on cell culture. The medium can be used for studying the differentiation and proliferation adjusting mechanism of the umbilical cord mesenchymal stem cells.
Owner:SHANDONG JINGYUAN BIOTECH CO LTD
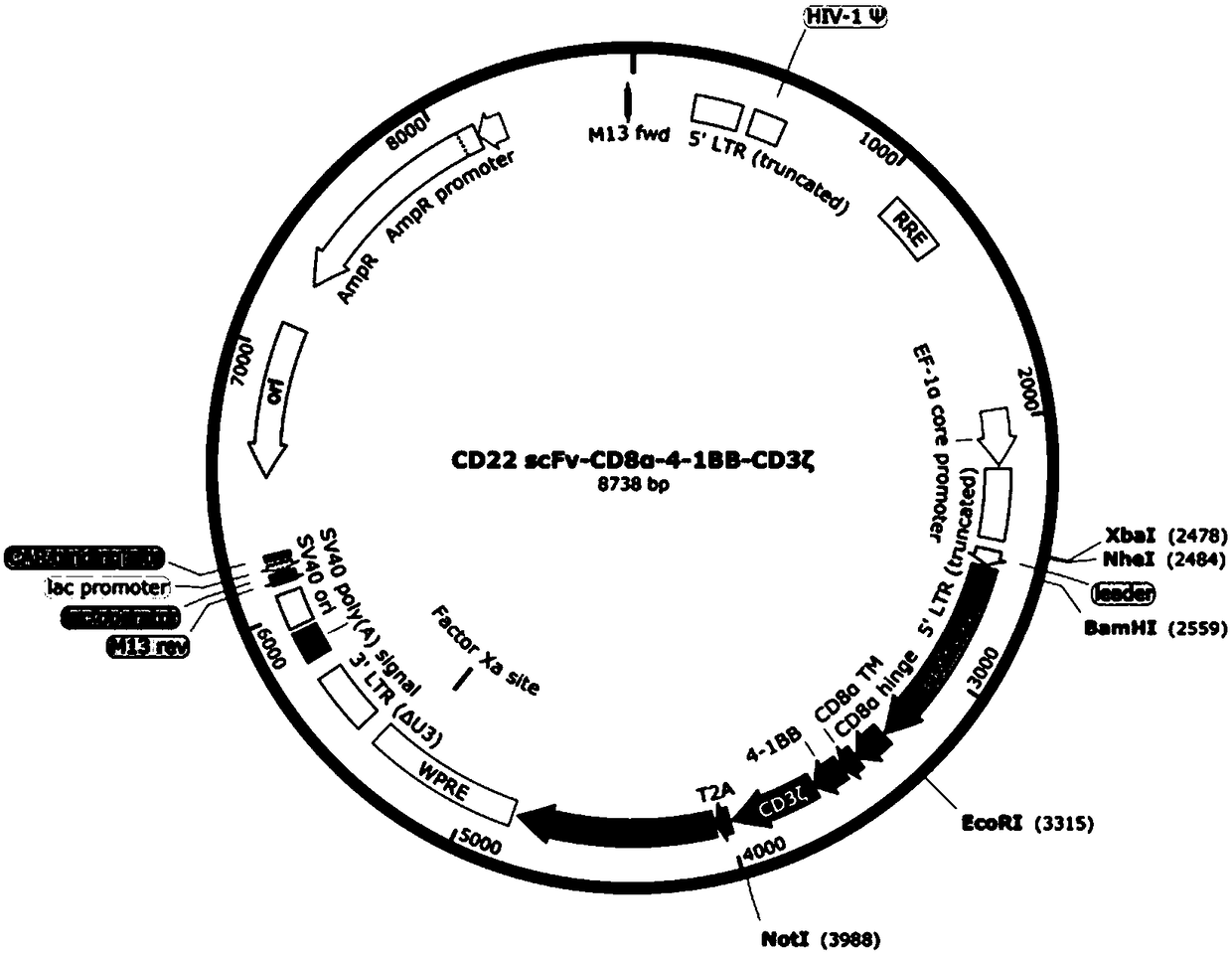
Chimeric antigen receptor targeting CD22 and application of chimeric antigen receptor
ActiveCN108715859AGood killing effectNo lethal effectPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenOn cells
The invention provides a nucleic acid molecule for encoding a chimeric antigen receptor targeting CD22. The chimeric antigen receptor comprises an extracellular region, a transmembrane region and a intracellular signal transduction region, the extracellular region encoded by the nucleic acid molecule comprises a CD22 binding domain, and the CD22 binding domain is amino acid sequence as shown in SEQ ID No. 3. By using flow cytometry, a degranulation analysis experiment and ELISA to detect cell factors secreted by a T cell, a fact that the CAR-T cell has a high lethal effect on B cell lymphoma cells and acute B cell lymphoma cell leukemia cells expressing CD22 and hardly has a lethal effect on cells which do not express CD22, and an off-target effect is prevented effectively is proved. The chimeric antigen receptor CD22scFv-CD8alpha-4-1BB-CD3zeta can be used for treating CD22<+>B cell hematologic neoplasms and is applicable to combined treatment with a CD19 CAR-T cell.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
Chimeric antigen receptor
InactiveUS20170066827A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorBinding domain
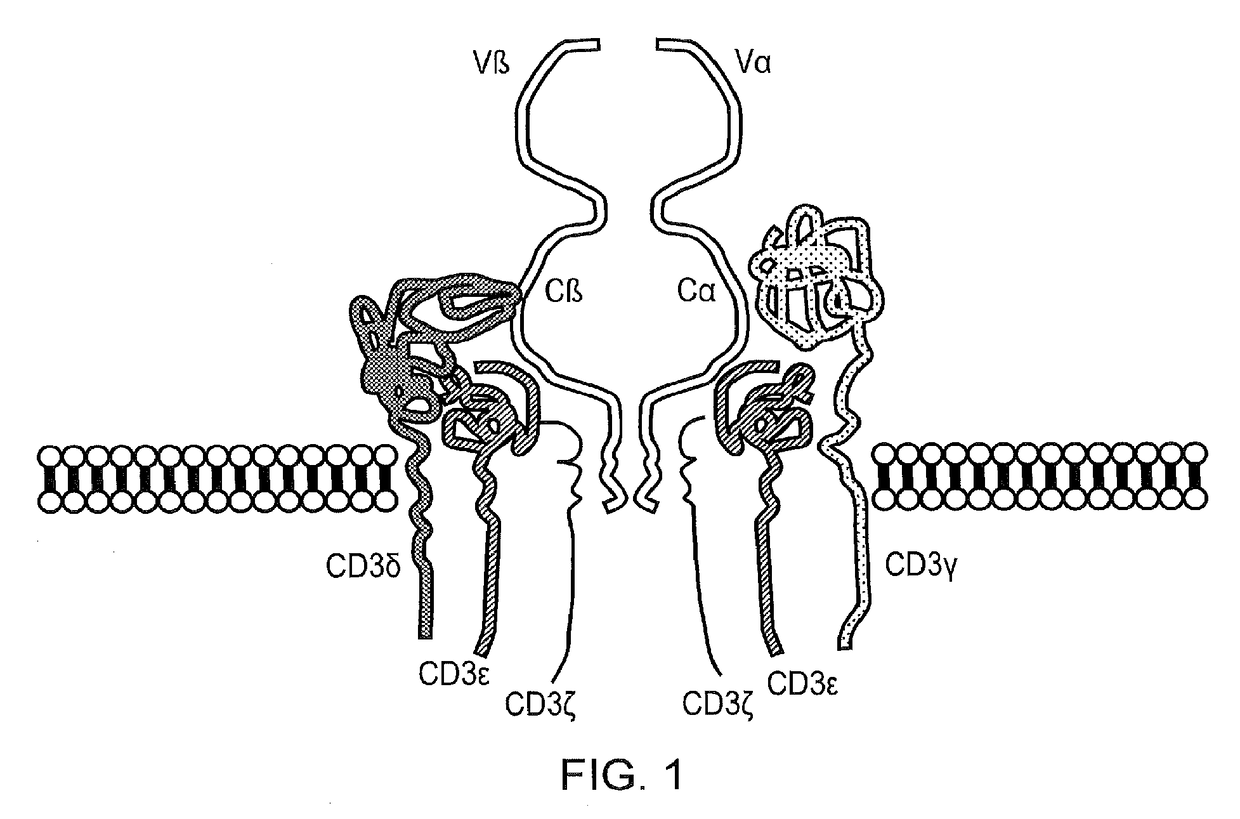
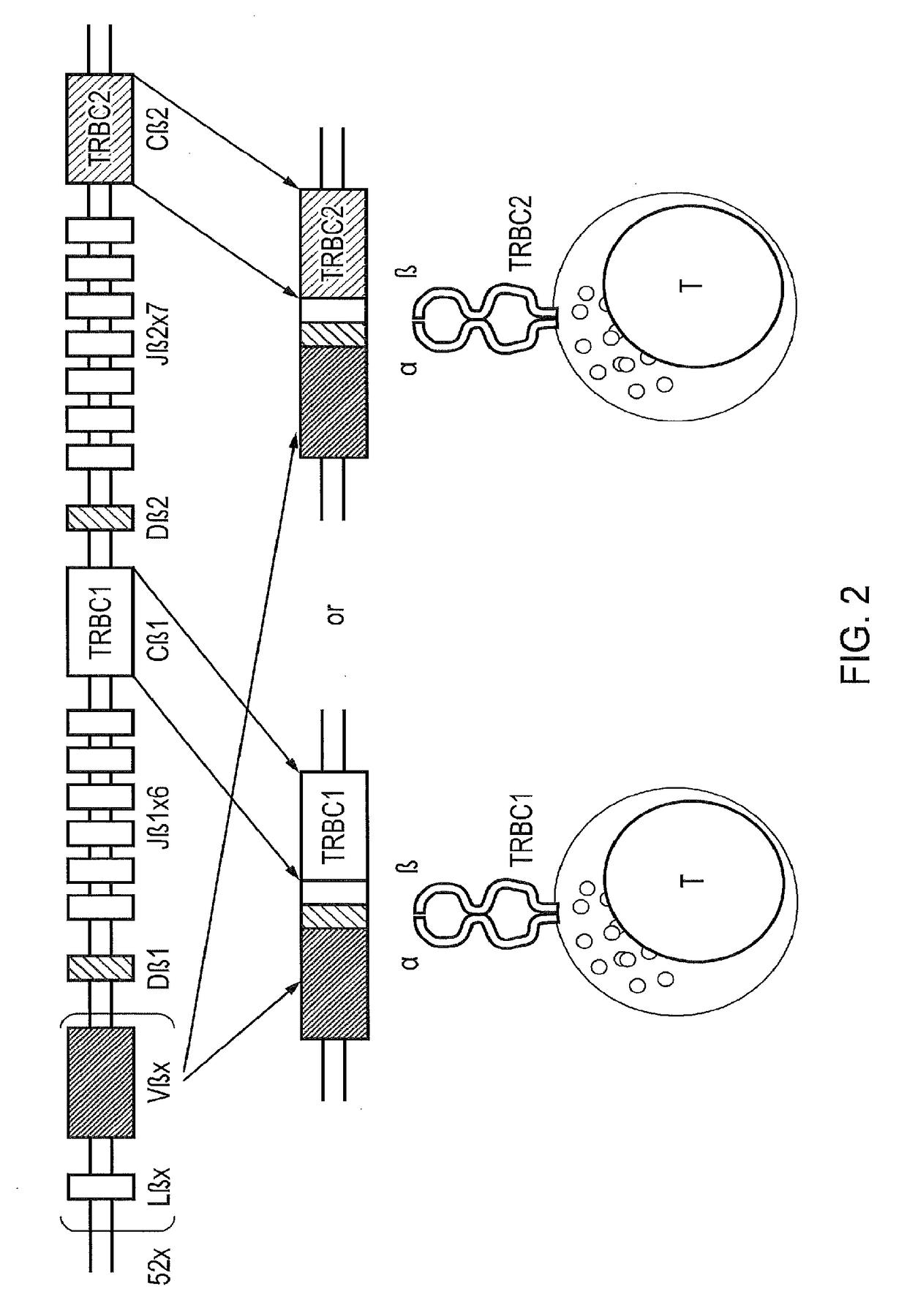
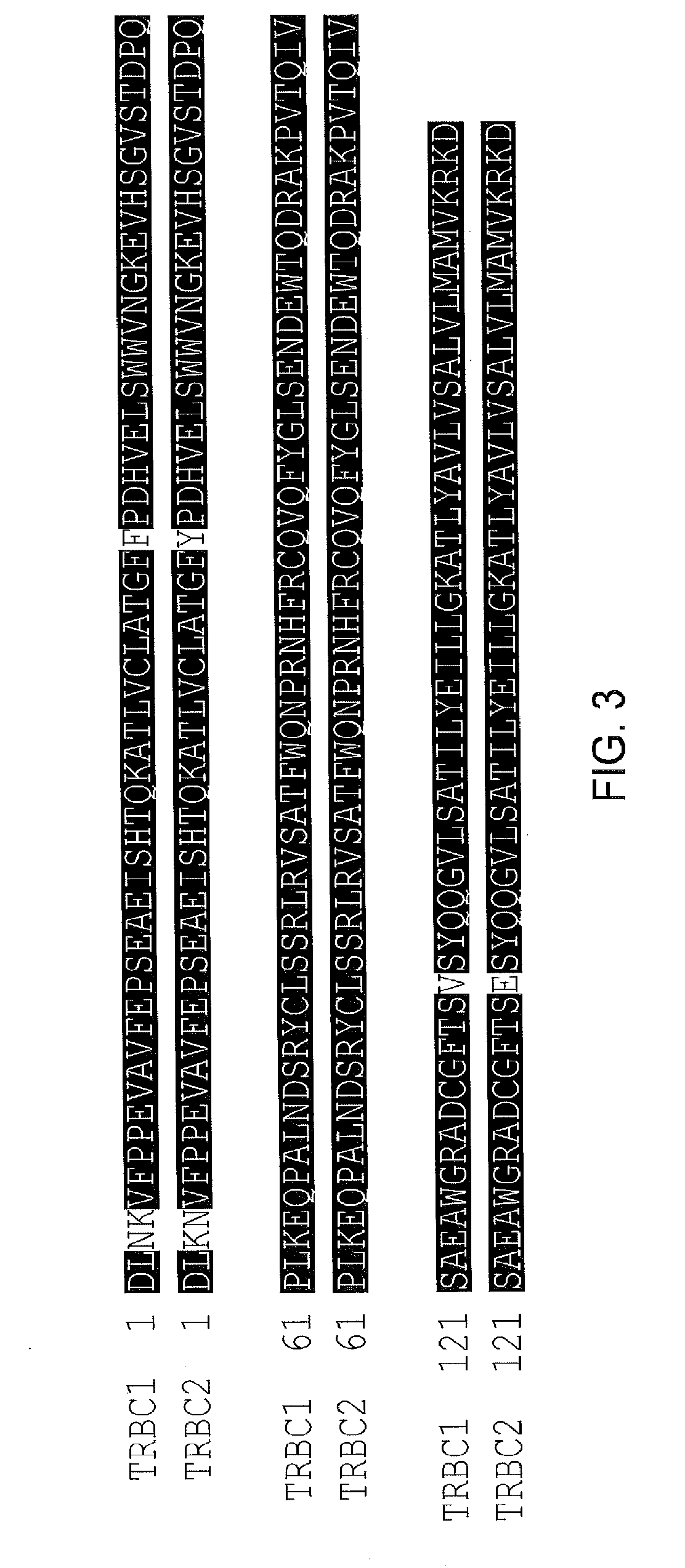
The present disclosure relates to a chimeric antigen receptor (CAR) which comprises an antigen-binding domain which selectively binds TCR beta constant region 1 (TRBC1) or TRBC2; cells; such a T cells comprising such a CAR; and the use of such cells for the treatment of a T-cell lymphoma or leukaemia in a subject.
Owner:AUTOLUS LIMIED
Cytotoxic Antibody Directed Against Type B Lymphoid Hematopoietic Proliferations
ActiveUS20090053233A1Reduced dosLow immunogenicityImmunoglobulins against cell receptors/antigens/surface-determinantsImmunological disordersCD20Cytotoxic antibody
The present invention relates to a monoclonal antibody directed against the CD20 antigen, wherein the variable region of each of the light chains thereof is encoded by a sequence which shares at least 70% identity with murine nucleic acid sequence SEQ ID No. 5, the variable region of each of the heavy chains thereof is encoded by a sequence which shares at least 70% identity with murine nucleic acid sequence SEQ ID No. 7, and the constant regions of light and heavy chains thereof are constant regions from a non-murine species, as well as for activation of FcγRIIIA receptors in immune effector cells, and for the manufacture of a drug especially for the treatment of leukaemia or lymphoma.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Chimeric antigen receptor T cells targeting CD19, and application of chimeric antigen receptor T cells
InactiveCN108276497AInhibit tumor cell proliferationActivate the killing mechanismMammal material medical ingredientsImmunoglobulinsNH lymphomaInterleukin 2
The invention discloses chimeric antigen receptor T cells targeting CD19, and application of the chimeric antigen receptor T cells. A chimeric antigen receptor for preparing the chimeric antigen receptor T cells comprises interleukin 2 signal peptide, an anti-CD19 single chain antibody, a CD8 protein molecular hinge region, a transmembrane region, an intracellular signal structural domain, and anintracellular signal transduction structural domain of CD3 zeta protein molecules which are sequentially connected in series. The chimeric antigen receptor T cells are used for preparing medicines orpreparations for treating hematological malignancies, wherein the hematological malignancies comprise CD19-positive acute B-lymphocytic leukemia, diffuse large B-cell lymphoma and non-Hodgkin lymphoma.
Owner:英普乐孚生物技术(上海)有限公司
Cd123-specific chimeric antigen receptor redirected t cells and methods of their use
A family of chimeric antigen receptors (CARs) containing a CD123 specific scFv was developed to target different epitopes on CD123. In some embodiments, such a CD123 chimeric antigen receptor (CD123CAR) gene includes an anti-CD123 scFv region fused in frame to a modified IgG4 hinge region comprising an S228P substitution, an L235E substitution, and optionally an N297Q substitution; a costimulatory signaling domain; and a T cell receptor (TCR) zeta chain signaling domain. When expressed in healthy donor T cells (CD4 / CD8), the CD123CARs redirect T cell specificity and mediated potent effector activity against CD123+ cell lines as well as primary AML patient samples. Further, T cells obtained from patients with active AML can be modified to express CD123CAR genes and are able to lyse autologous AML blasts in vitro. Finally, a single dose of 5.0 x 106 CAR123 T cells results in significantly delayed leukemic progression in mice. These results suggest that CD123CAR-transduced T cells may be used as an immunotherapy for the treatment of high risk AML.
Owner:CITY OF HOPE
CAR-T (chimeric antigen receptor T cell) for targeting CD19 and application of CAR-T
ActiveCN107827991ARestrict growthPrevent proliferationMammal material medical ingredientsImmunoglobulinsSequence signalSingle-Chain Antibodies
The invention discloses a CAR-T (chimeric antigen receptor T cell) for targeting CD19 and an application of the CAR-T. A CAR for preparing the CAR-T comprises interleukin 2 signal peptides, an anti-CD19 single-chain antibody, a CD8 protein molecule hinge region, a transmembrane region, an intracellular signal structure region and a CD3 zeta protein molecule intracellular signal conduction structure region which are connected in series sequentially and has the amino acid sequence shown in SEQ ID NO:9. The CAR-T is applied to preparation of a medicine or a preparation for treating hematologicalmalignancy, wherein the hematological malignancy comprises CD19-positive B-cell acute lymphocytic leukemia, diffuse large B cell lymphoma and non-hodgkin's lymphoma.
Owner:英普乐孚生物技术(上海)有限公司
Children acute lymphoblastic leukaemia genotyping diagnosis chip
InactiveCN101525667AReduce complicationsHigh cure rateNucleotide librariesMicrobiological testing/measurementLife qualityA-DNA
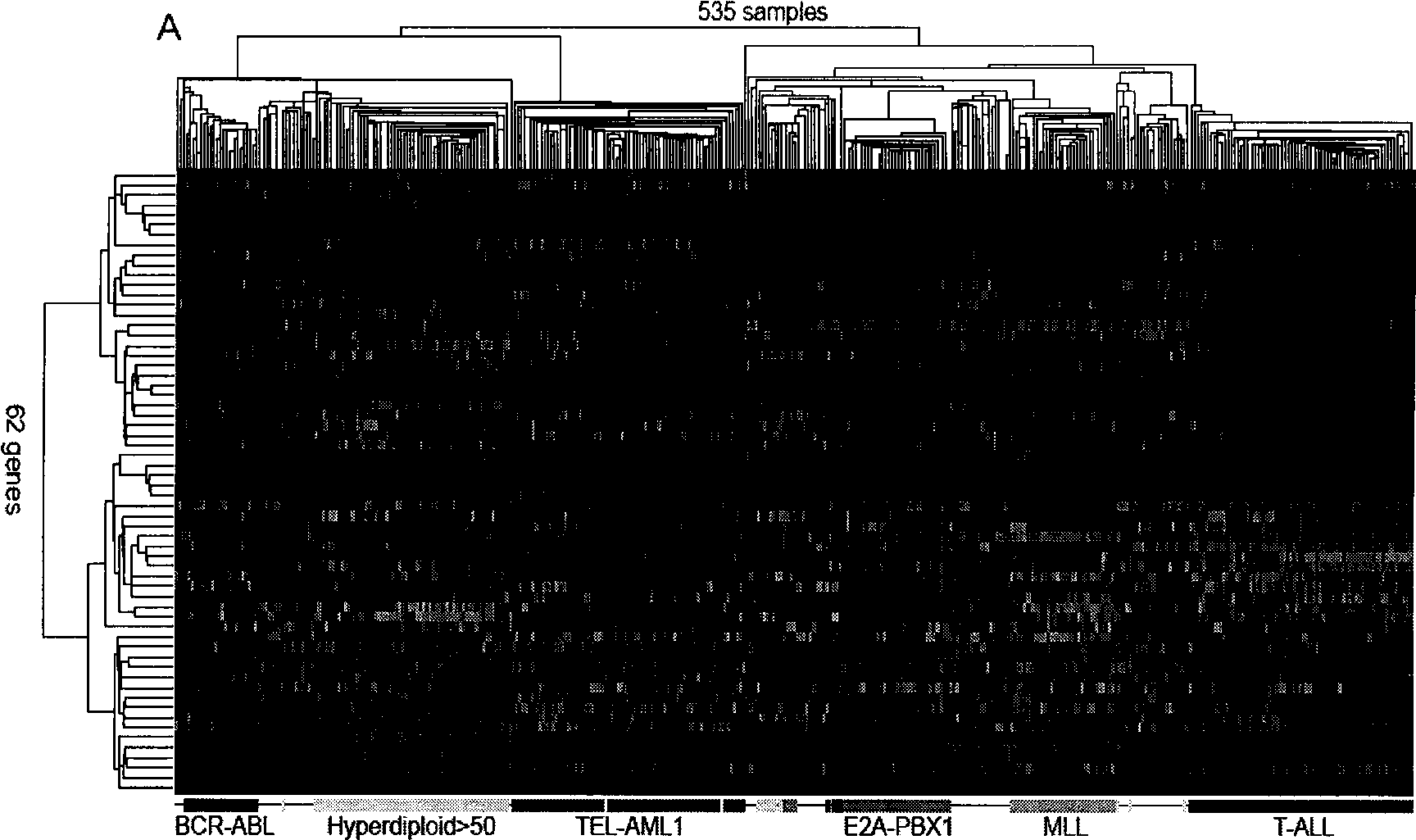
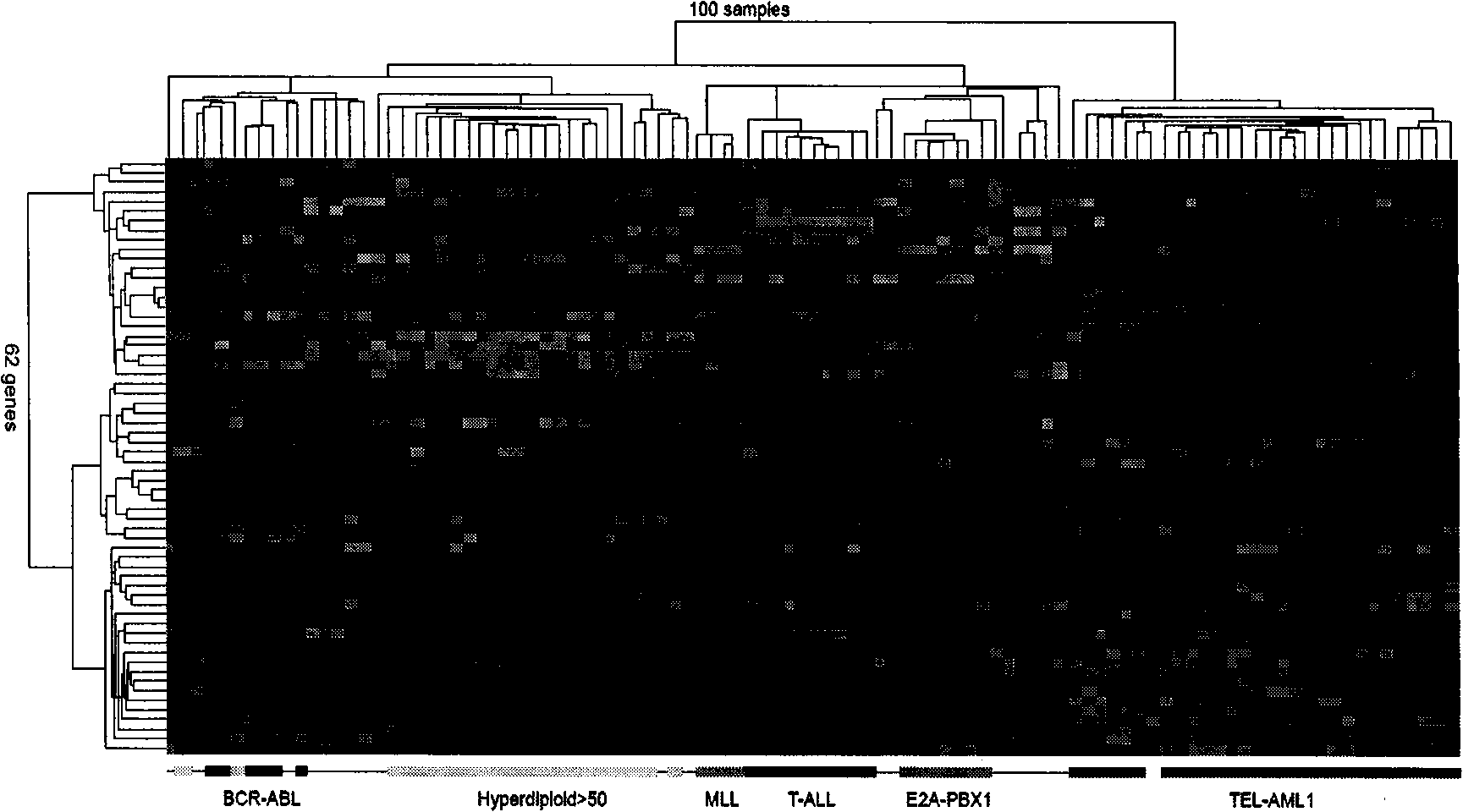
The invention discloses a children acute lymphoblastic leukaemia genotyping diagnosis chip. The children acute lymphoblastic leukaemia genotyping diagnosis chip is a DNA chip which is fixed with 62 DNA fragment arrays on the surface of a carrier. .The nucleotide sequences of the 62 DNA fragment arrays are respectively the sequence 1 to the sequence 62 of the sequence list. The invention further discloses a children acute lymphoblastic leukaemia genotyping method. The genetic chip of the invention can provide precise typing so as to help correctly choose a chemo-treatment plan of appropriate strength, thereby reducing complicating disease and improving curative ratio and life quality. The genetic chip of the invention can be used to carry out genotyping on childhood ALL. Therefore, the genetic chip not only saves diagnosis cost, but also has significance in protecting labour resource and promoting family and society harmony.
Owner:BEIJING CHILDRENS HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV +1
Chimeric antigen receptor of targeted CD19 as well as method and use for jointly expressing IL-15
ActiveCN108728459AMammal material medical ingredientsNucleic acid vectorAntiendomysial antibodiesTumor cells
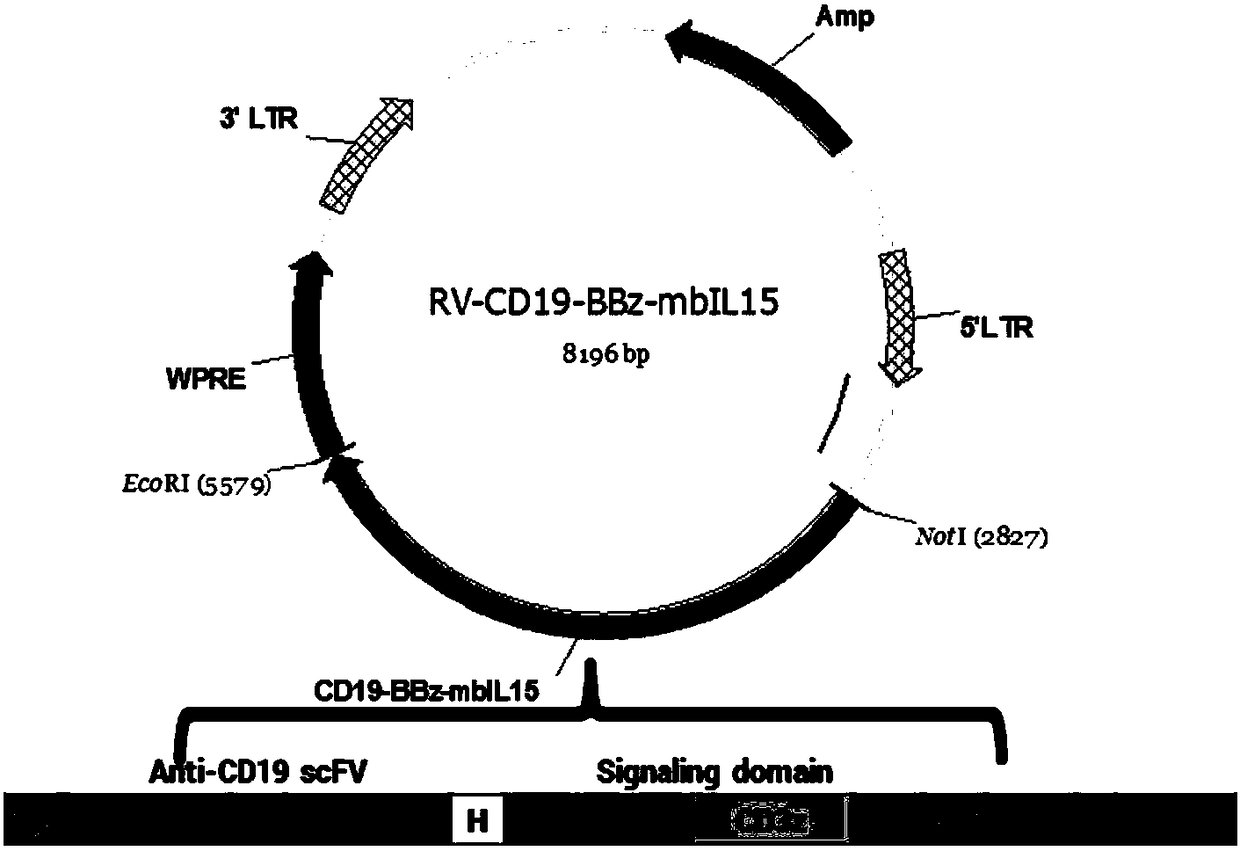
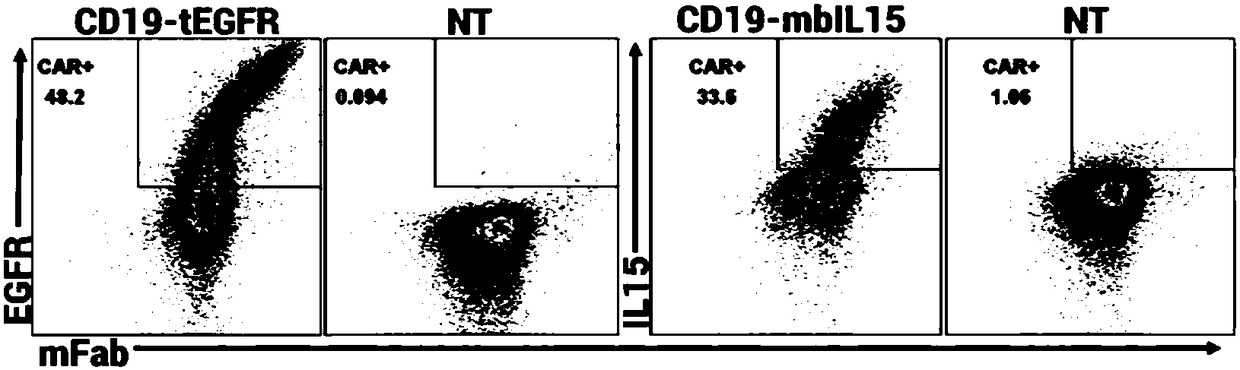
The invention discloses a chimeric antigen receptor CD19-CD8H&TM-41BB-CD3zeta-mbIL15 and use thereof. The chimeric antigen receptor is formed by series connection of a heavy chain and light chain variable region (CD19scFV) of a mouse anti-human CD19 monoclonal antibody (with a cloning number being FMC63), a human CD8alpha hinge region, a transmembrane region, a human 41BB intracellular region, a human CD3zeta intracellular region and an IL15+IL15Ralphla structure. The chimeric antigen receptor is used for modifying a human T-lymphocyte, and the modified T-cell (CAR-T cell) can be used for expressing CD19 positive acute / chronic lymphocytic leukemia and treating lymphoma. The prepared CD19 mbIL15 CAR-T cell has a strong killing function on specific tumor cells.
Owner:HRAIN BIOTECHNOLOGY CO LTD
Methods for culturing human myeloid leukaemia cells and cells derived therefrom
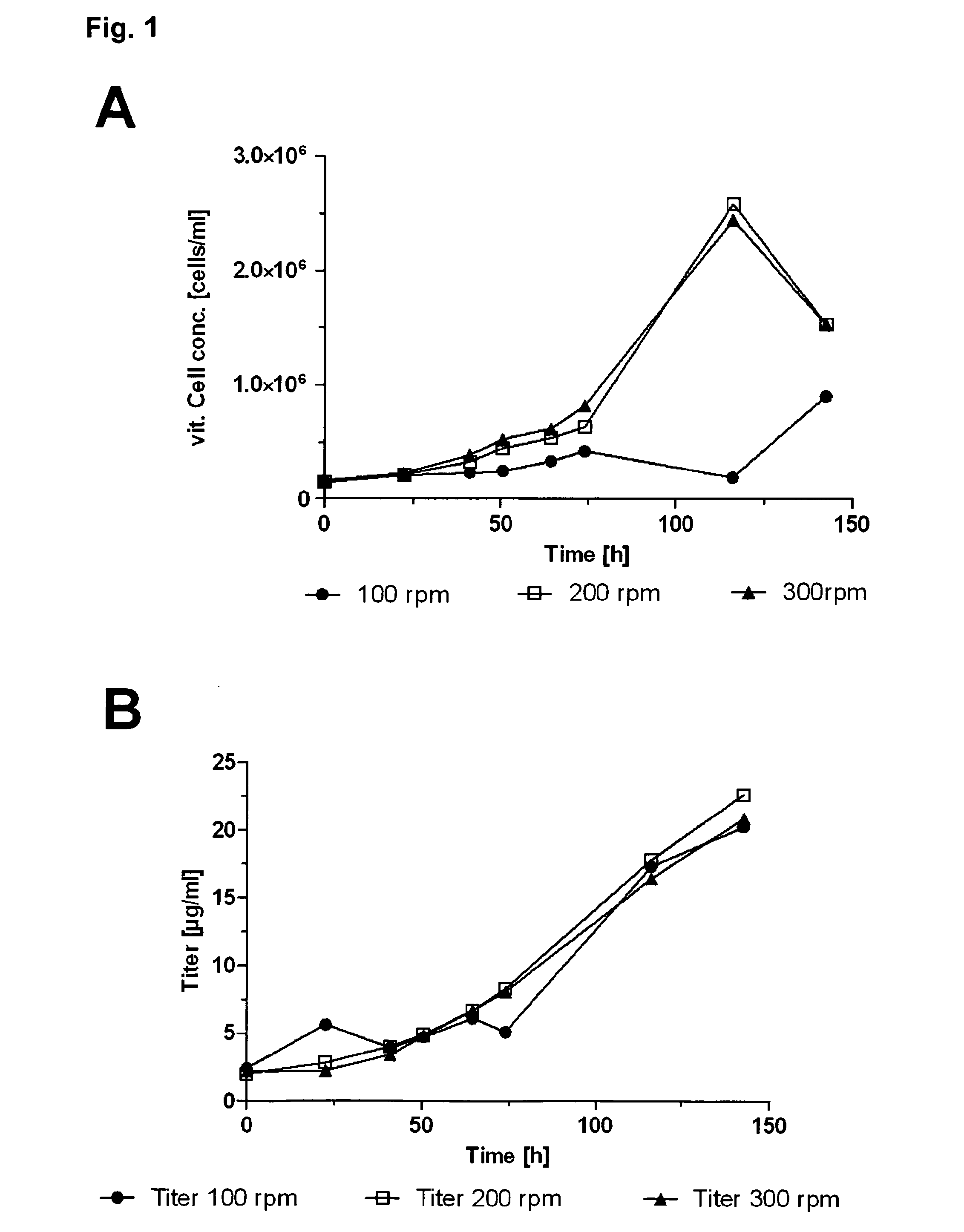
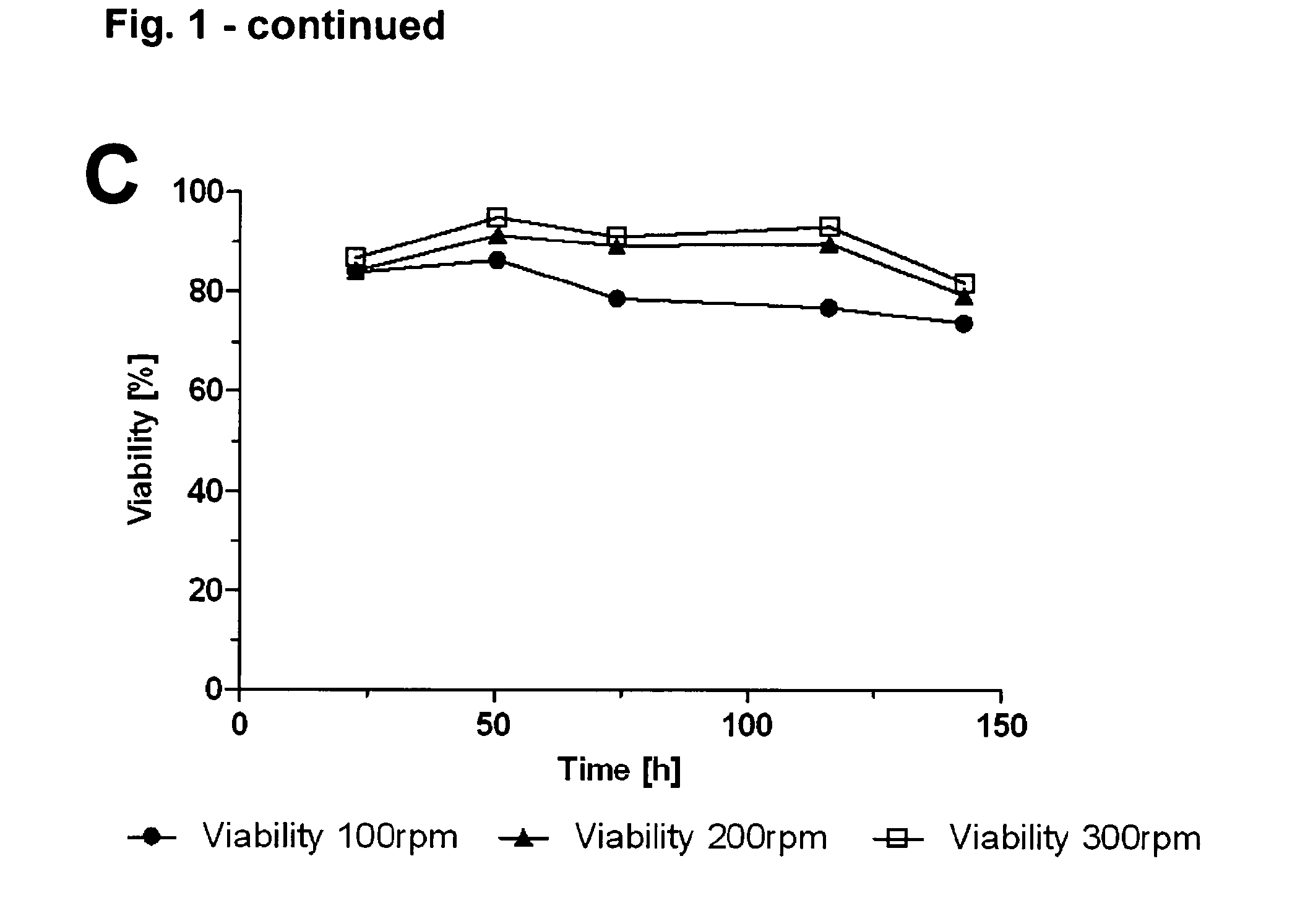
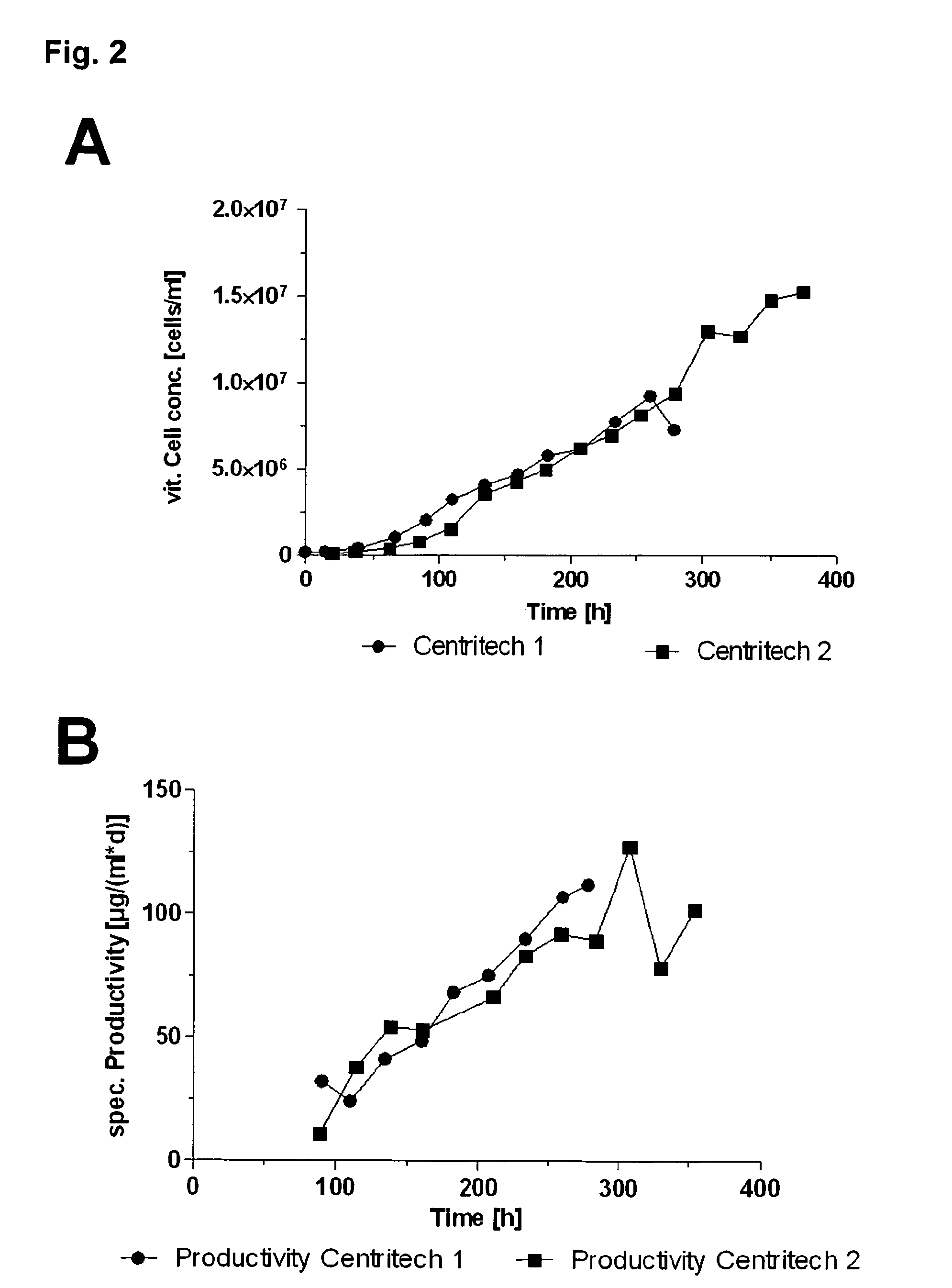
InactiveUS20130330768A1Enhance stirringHigh yieldCulture processArtificial cell constructsBiological immortalityCell biology
The present invention pertains to a method for culturing a suspension of immortalized human blood cells, preferably cells of myeloid leukaemia origin or cells derived therefrom, wherein said method provides a high productivity, a high cell viability and growth rate and a high batch-to-batch consistency, and can be scaled up without altering these parameters.
Owner:GLYCOTOPE GMBH
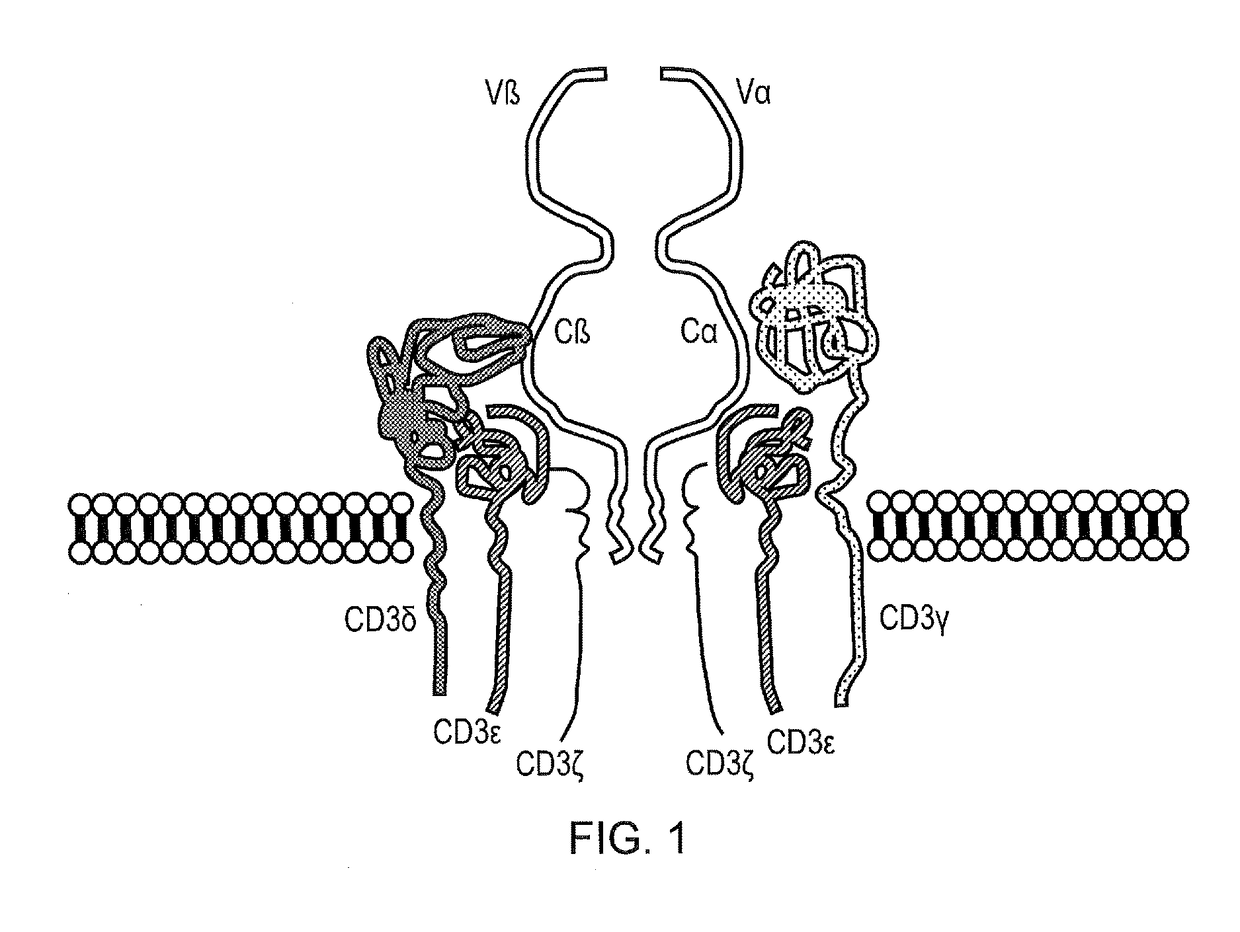
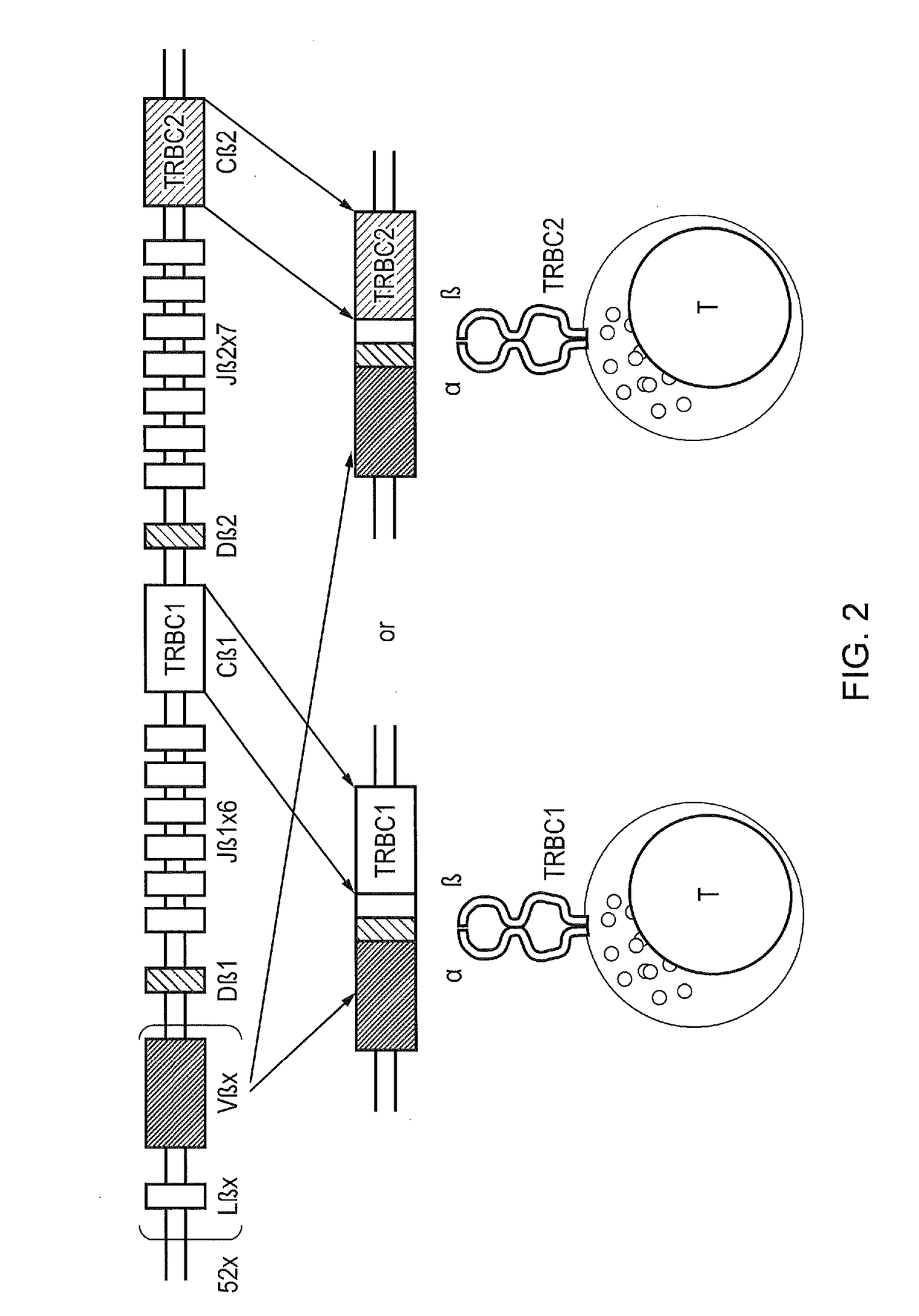
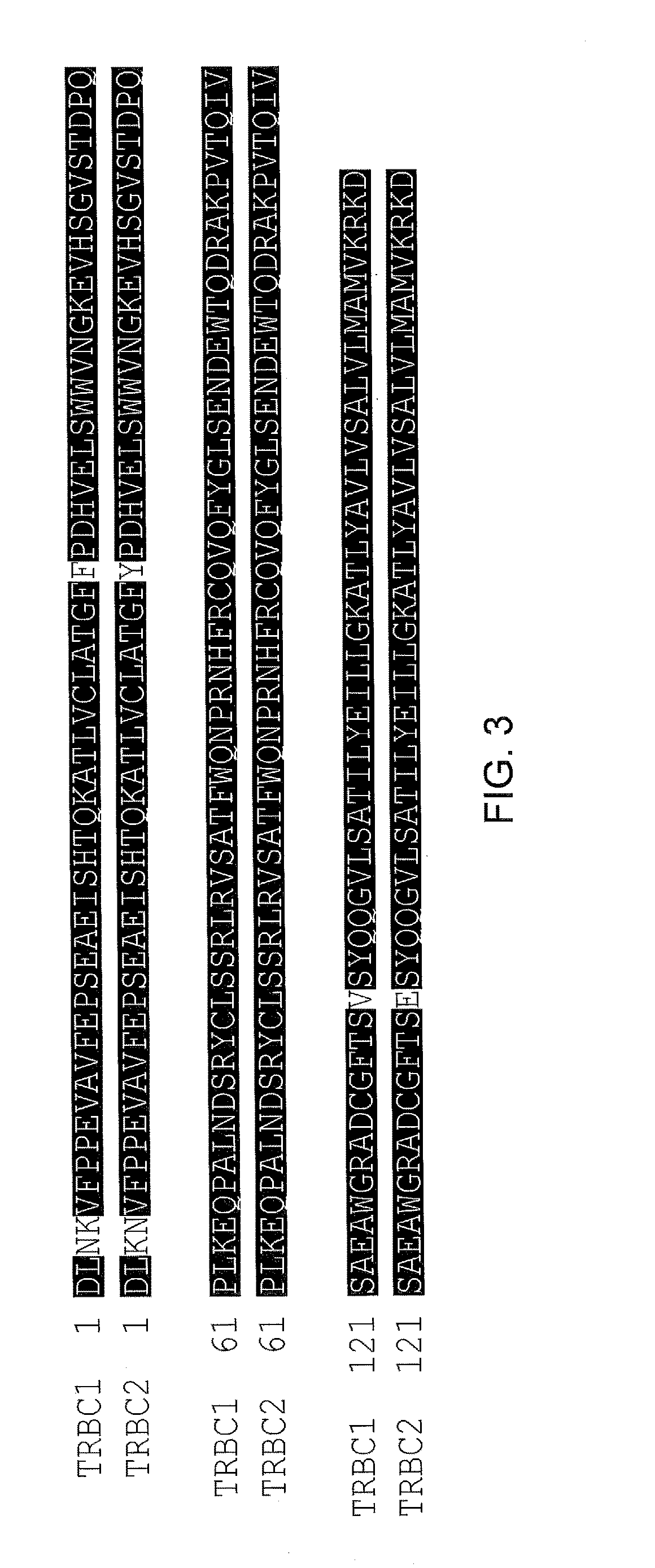
Chimeric antigen receptor (CAR) with antigen binding domains to the t cell receptor beta constant region
InactiveUS20170334998A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorT-Cell Receptor Beta
The present disclosure relates to a chimeric antigen receptor (CAR) which comprises an antigen-binding domain which selectively binds TCR beta constant region 1 (TRBC1) or TRBC2; cells; such a T cells comprising such a CAR; and the use of such cells for the treatment of a T-cell lymphoma or leukaemia in a subject.
Owner:AUTOLUS LIMIED
Preparation method of dihydroartemisinin liquid preparation
InactiveCN101732250AGood dispersionGood water solubilityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityPEG 400
The invention discloses a preparation method of a dihydroartemisinin liquid preparation, which belongs to the field of medicine. The preparation method comprises the following steps: firstly, dissolving dihydroartemisinin into dimethyl sulfoxide, then adding ethanol and polyethylene glycol 400 and further diluting the dihydroartemisinin to obtain the dihydroartemisinin liquid preparation which can be mixed and dissolved with water in any proportion. Because of enhancing the dispersion degree of the dihydroartemisinin greatly, the water solubility of the dihydroartemisinin liquid preparation is obviously enhanced. The dihydroartemisinin liquid preparation is used as an anti-tumour preparation and can obviously suppress the growth and the multiplication of the cells of a leukaemia cell strain K562 which is cultured in vitro, so that the anti-tumour effect of the dihydroartemisinin liquid preparation is obviously enhanced.
Owner:林杨
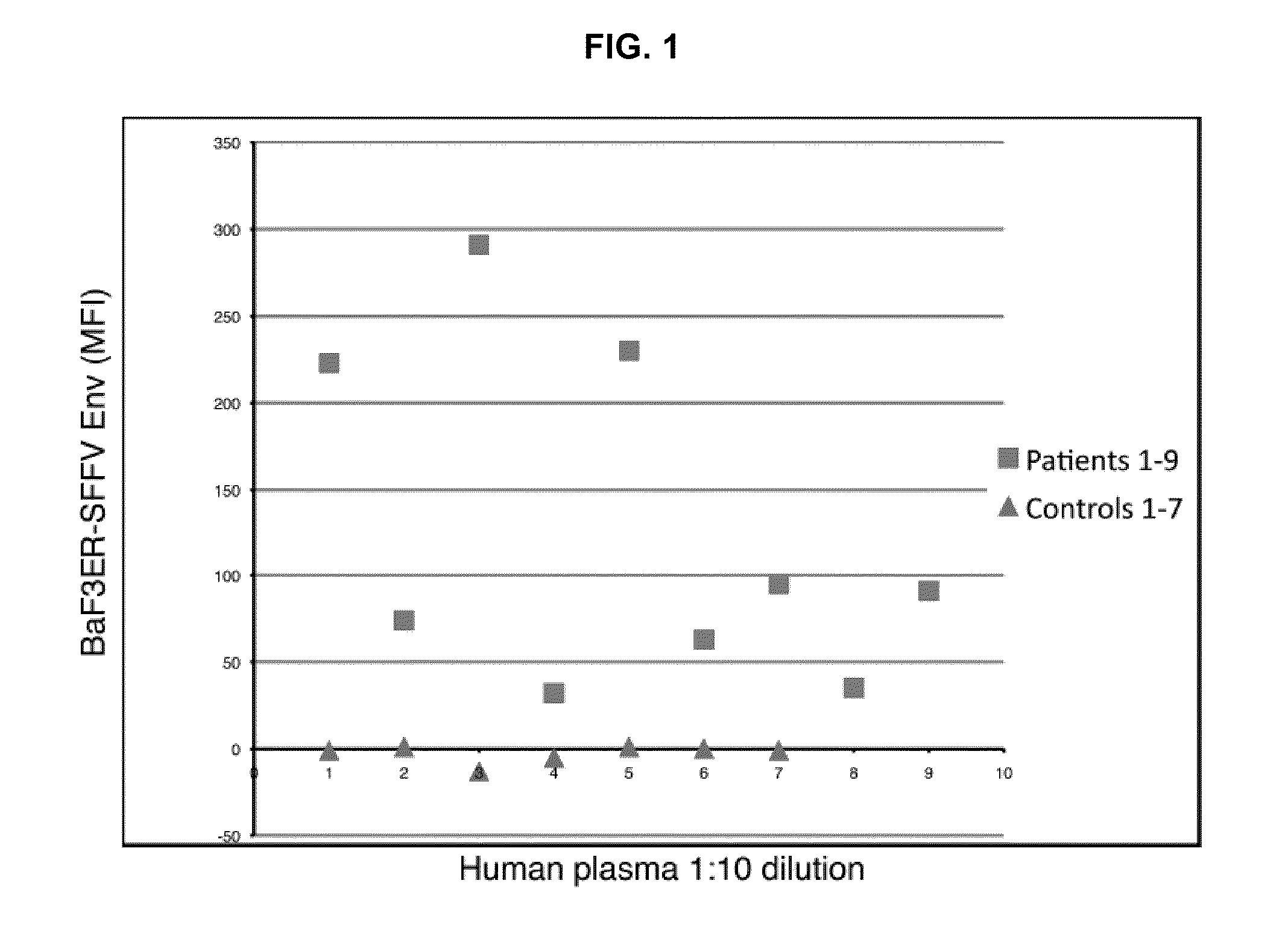
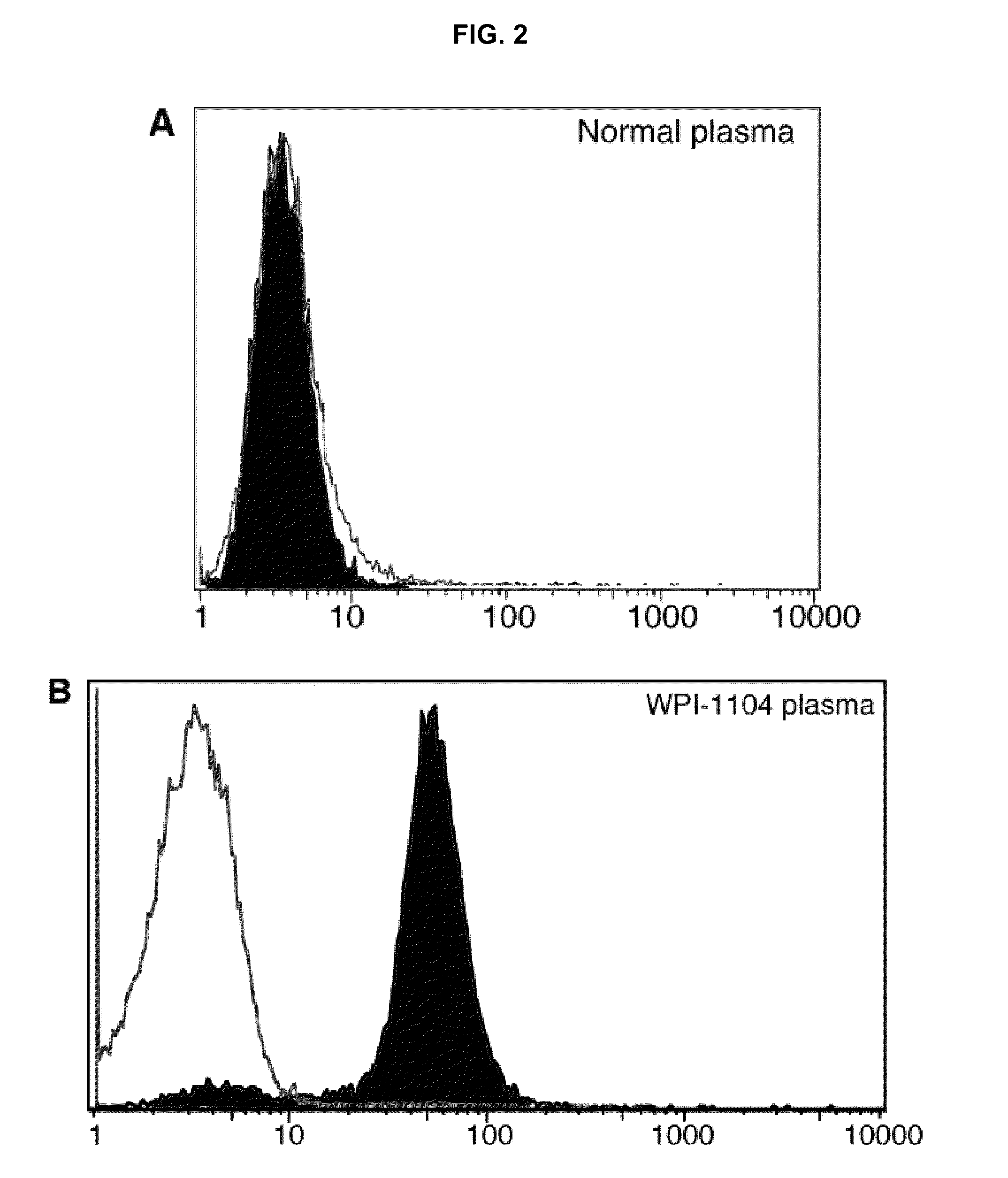
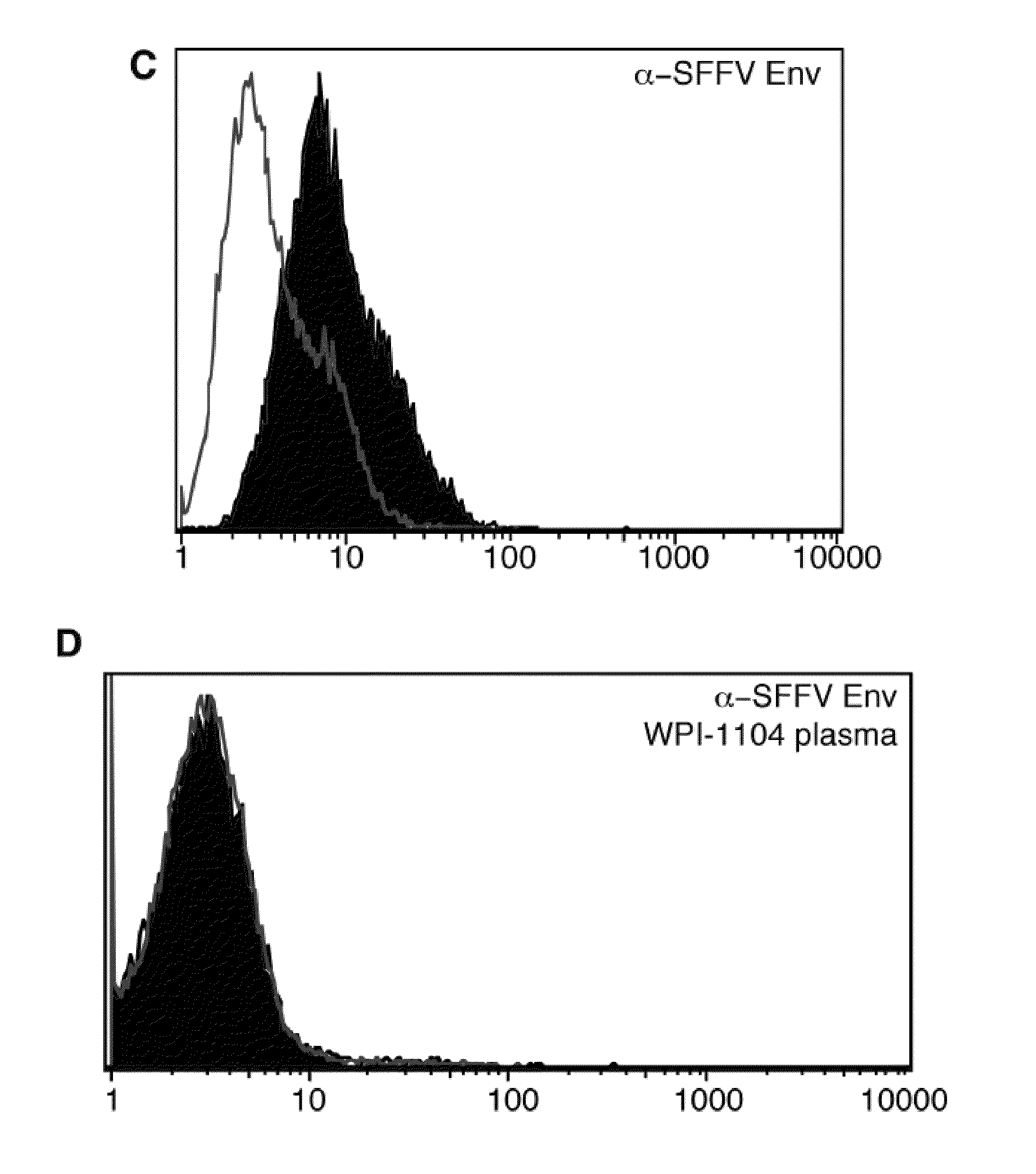
Seroconversion assays for detecting xenotropic murine leukemia virus-related virus
Methods of detecting, diagnosing, monitoring or managing an XMRV-related disease such as an XMRV-related neuroimmune disease such as chronic fatigue syndrome or an XMRV-related lymphoma such as mantle cell lymphoma in a subject are disclosed. These methods comprise determining presence, absence or quantity of antibodies against XMRV in a sample from a subject.
Owner:WHITTEMORE PETERSON INST FOR NEURO IMMUNE DISEASE
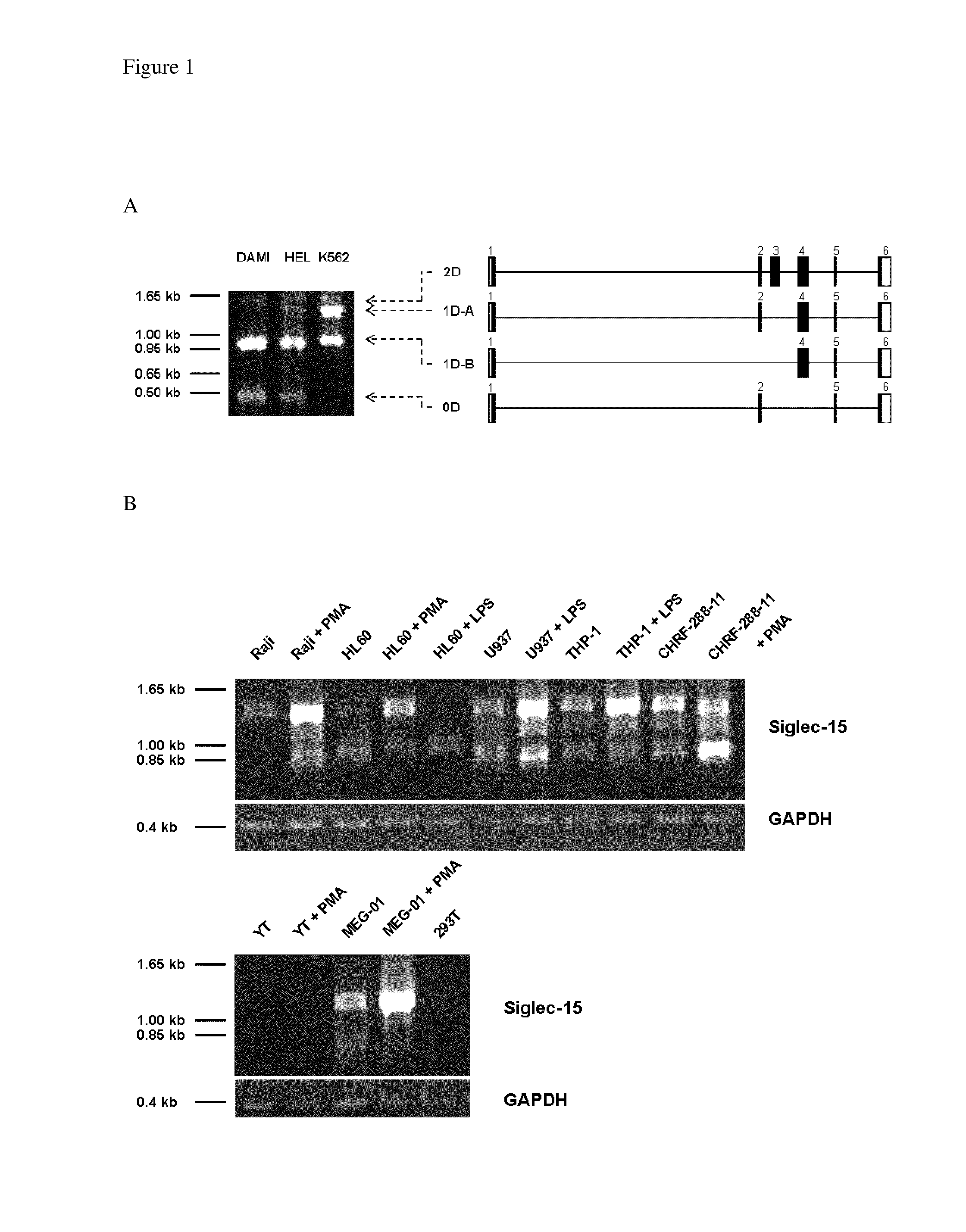
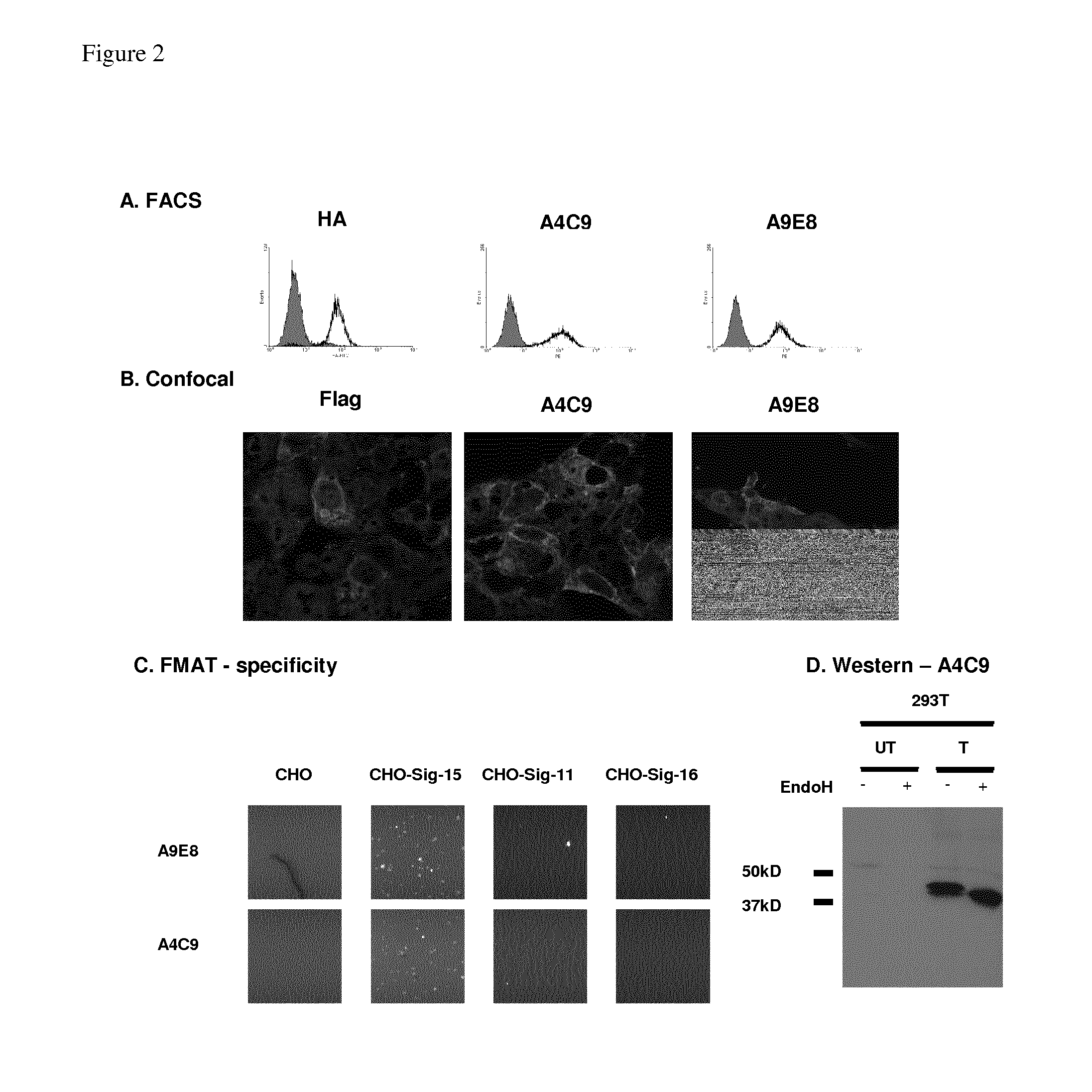
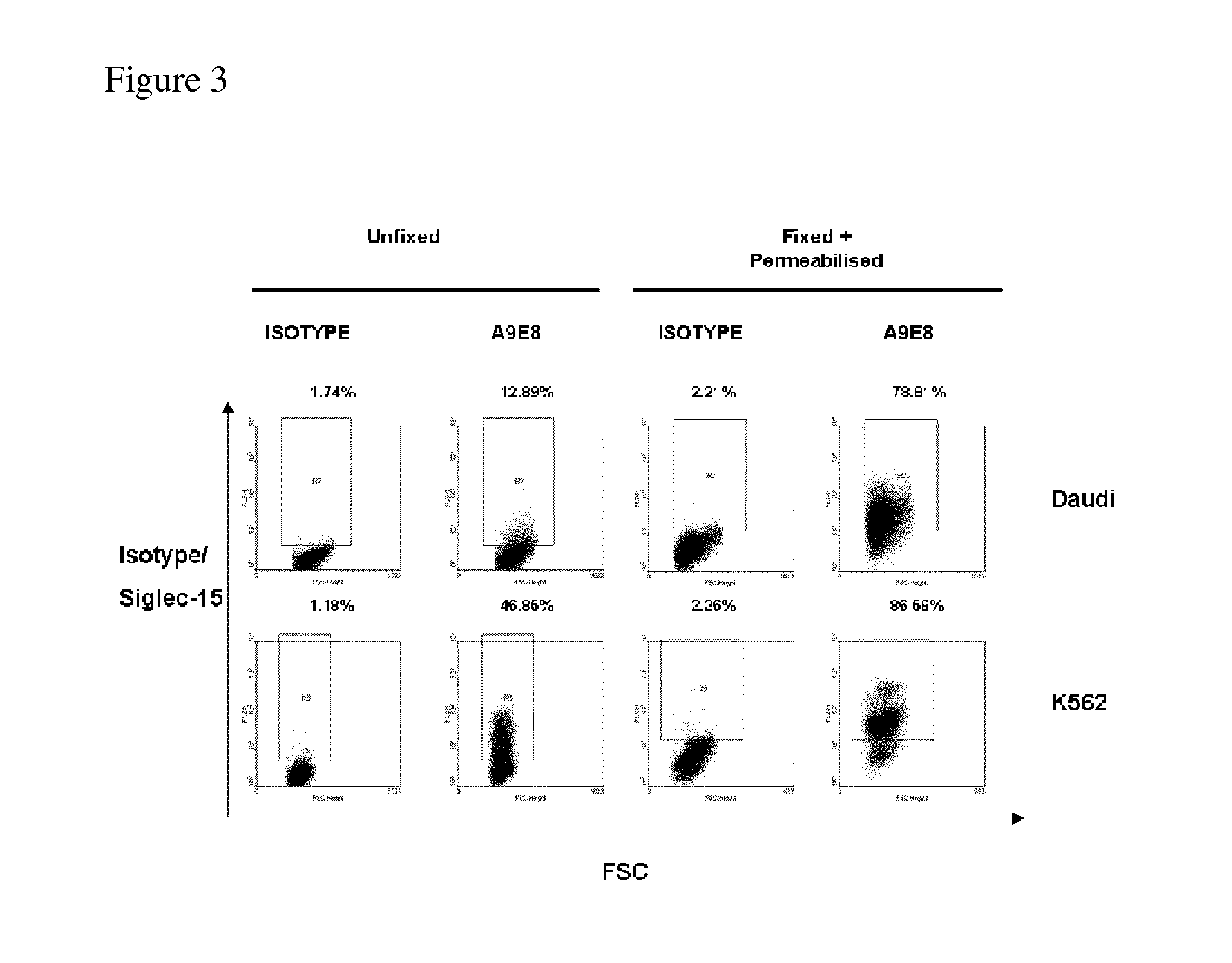
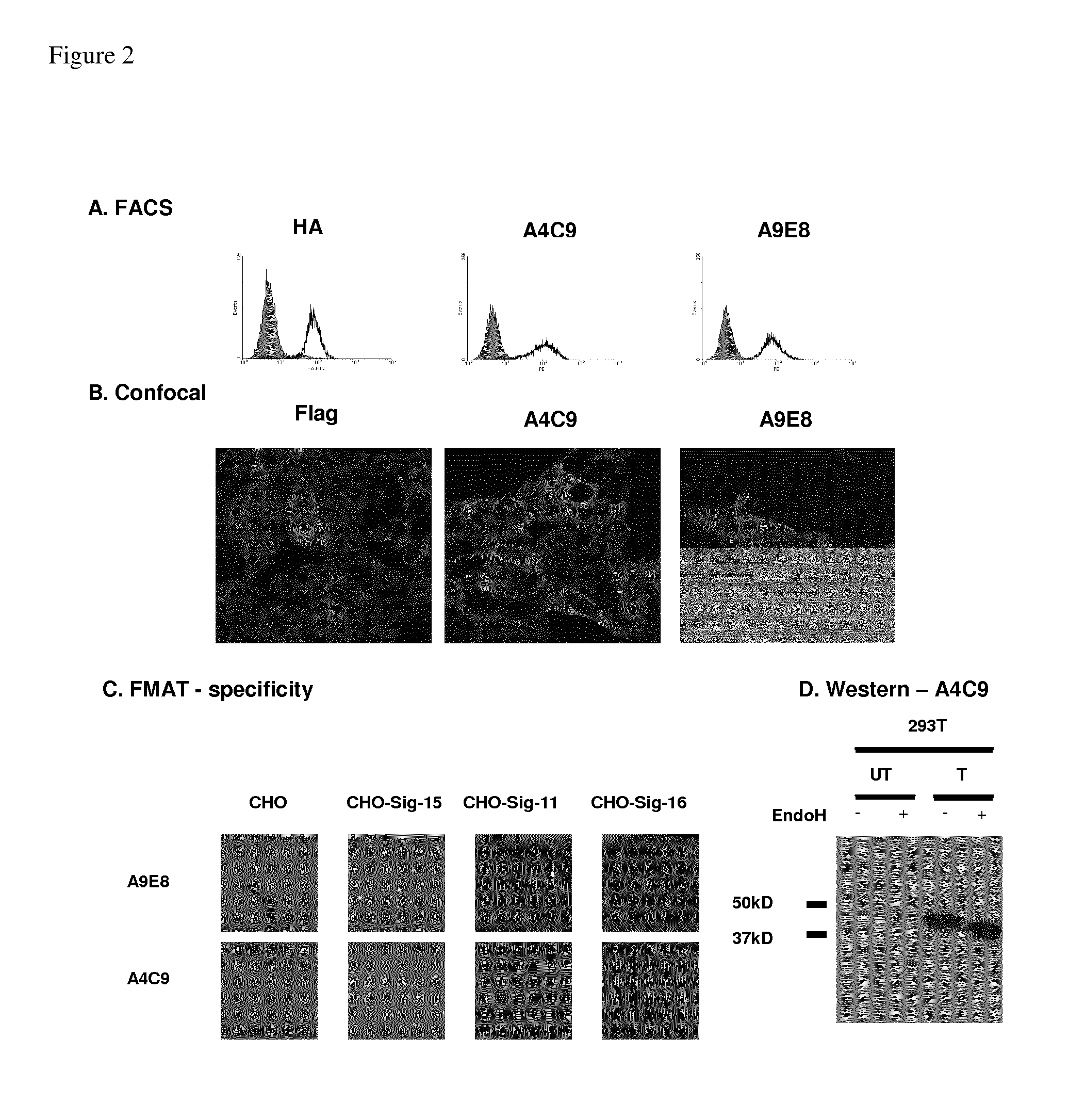
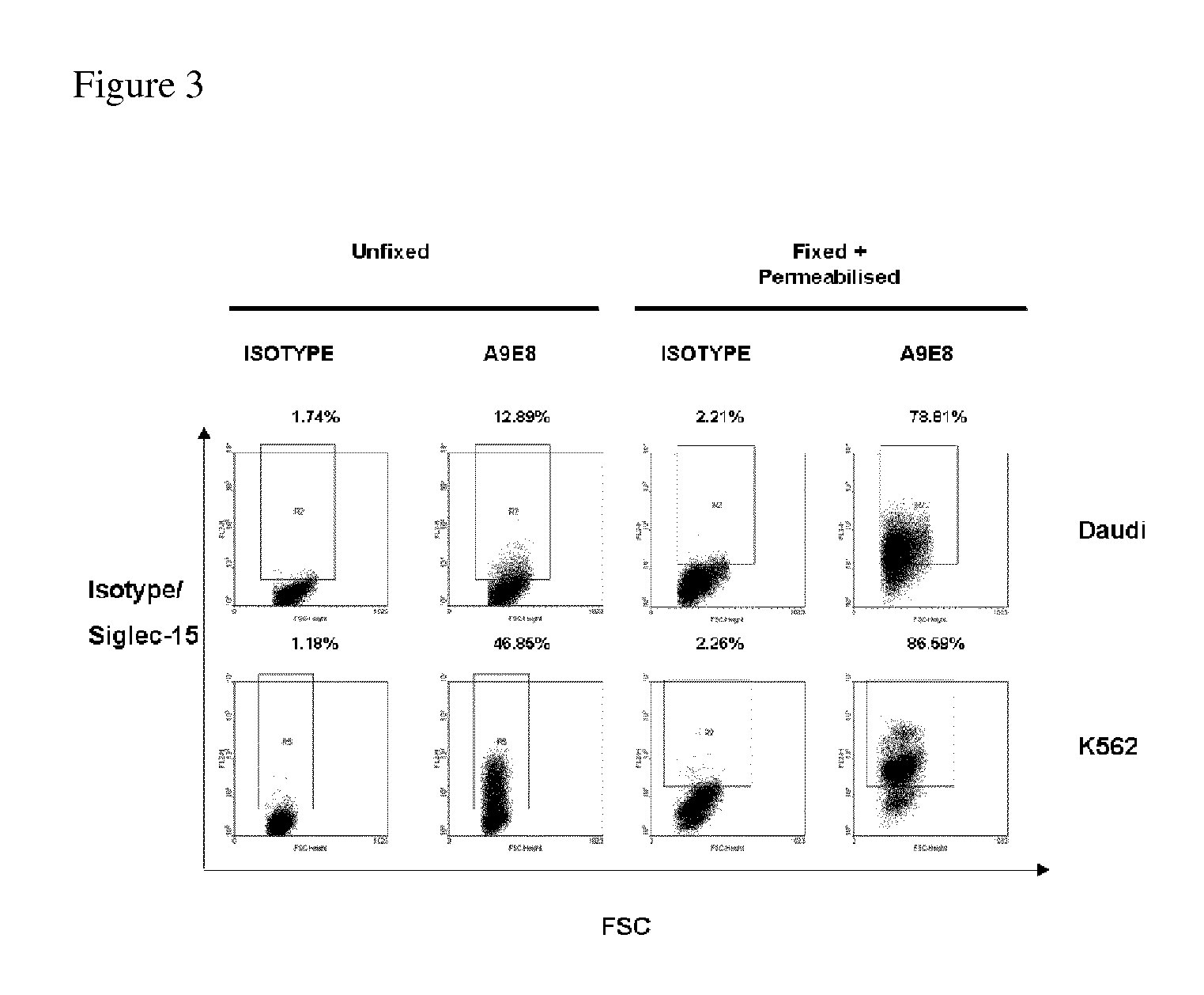
Anti-siglec-15 antibodies and uses thereof
ActiveUS20150037356A1Reduce in quantityEliminate side effectsAnimal cellsSugar derivativesAntibodyAML - Acute myeloid leukaemia
This disclosure relates to anti-Siglec-15 antibodies and uses thereof, in particular in the treatment of leukaemia, such as acute myeloid leukaemia.
Owner:MEDIMMUNE LTD +1
Compositions Comprising Human Embryonic Stem Cells and Their Derivatives, Methods of Use, and Methods of Preparation
The present invention relates to a pharmaceutical composition comprising of preparations of human embryonic stem (hES) cells and their derivatives and methods for their transplantation into the human body, wherein transplantation results in the clinical reversal of symptoms, cure, stabilization or arrest of degeneration of a wide variety of presently incurable and terminal medical conditions, diseases and disorders. The invention further relates to novel processes of preparing novel stem cell lines which are free of animal products, feeder cells, growth factors, leukaemia inhibitory factor, supplementary mineral combinations, amino acid supplements, vitamin supplements, fibroblast growth factor, membrane associated steel factor, soluble steel factor and conditioned media. This invention further relates to the isolation, culture, maintenance, expansion, differentiation, storage, and preservation of such stem cells.
Owner:SHROFF GEETA
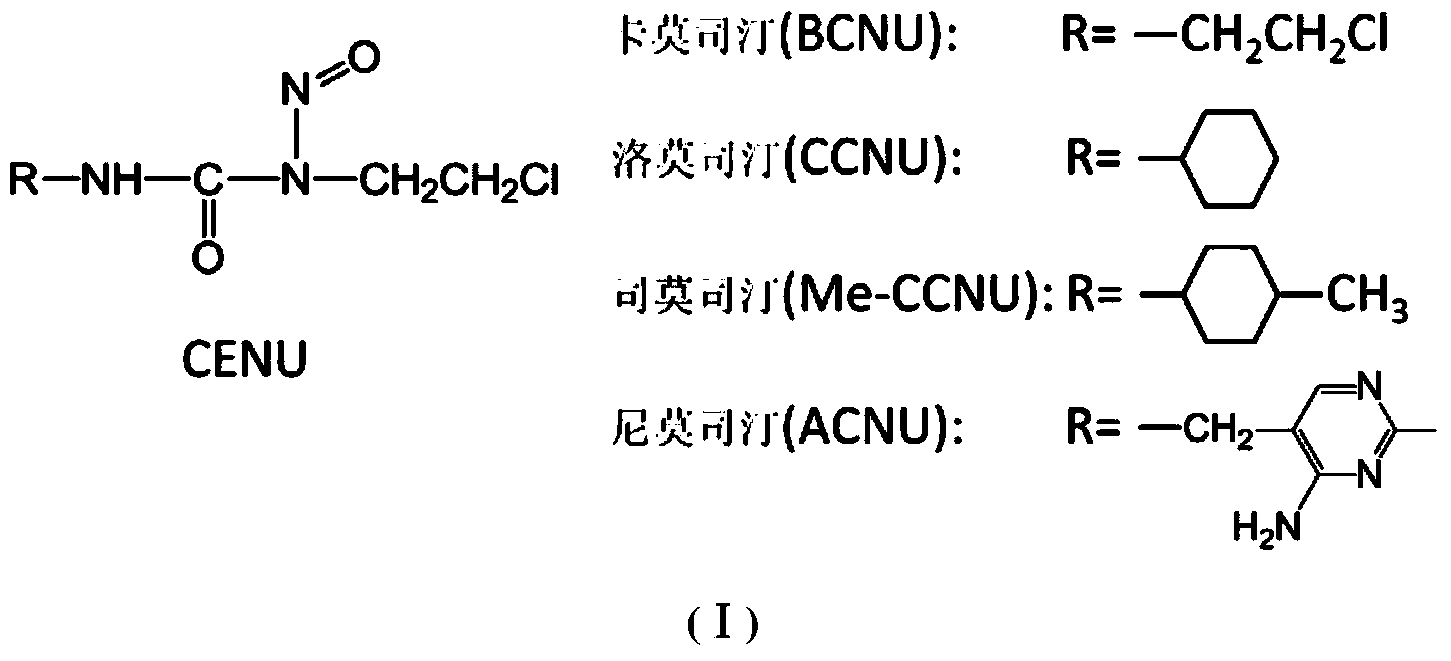
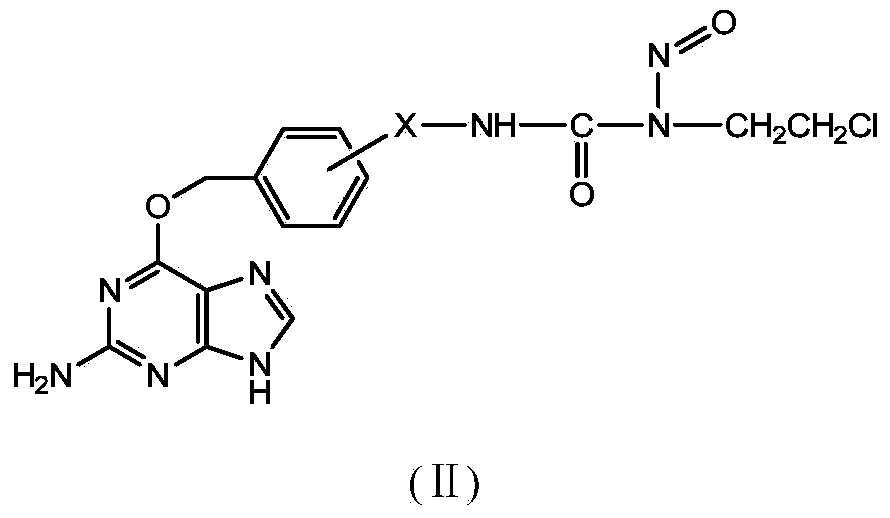
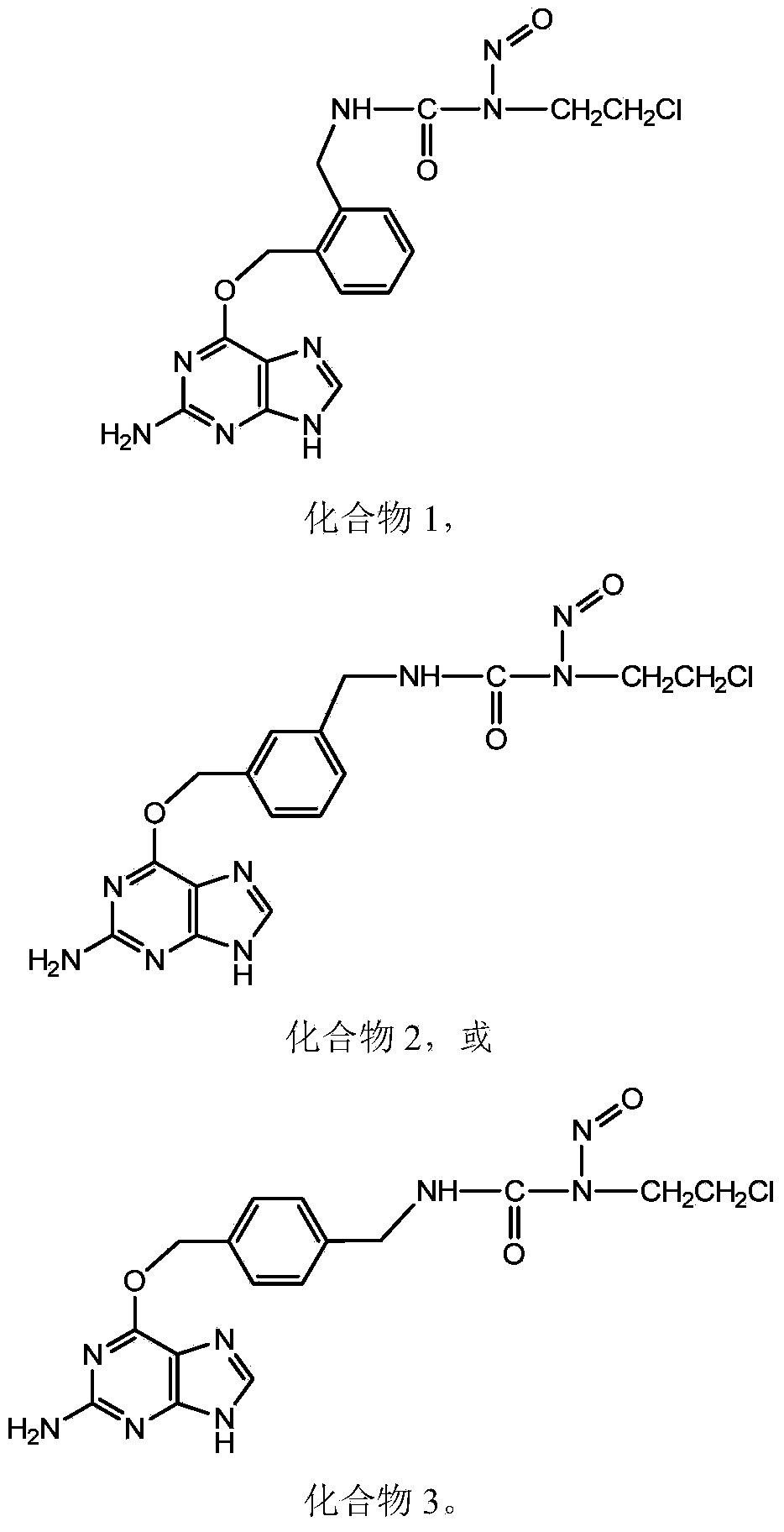
Novel beta-chloroethylnitrosourea compounds, and synthesis method and application thereof
ActiveCN104031048ADelay drug resistanceImprove targetingOrganic chemistryAntineoplastic agentsSynthesis methodsO6-Benzylguanine
The invention relates to novel beta-chloroethylnitrosourea compounds, and a synthesis method and application thereof. The structure of the beta-chloroethylnitrosourea compounds is disclosed as general formula (II). The in-vitro antitumor screening test on the compounds disclosed as general formula II proves that the compounds disclosed as general formula I have obvious inhibiting action on human cerebral nerve glioma cells SF763, SF767, SF126 and SF188, human colon cancer cell HT29, mouse leukaemia cell L1210 and many other tumor cell lines and have higher tumor inhibiting activity than the existing CENU and CENU / O6-benzylguanine combined medicine.
Owner:BEIJING UNIV OF TECH
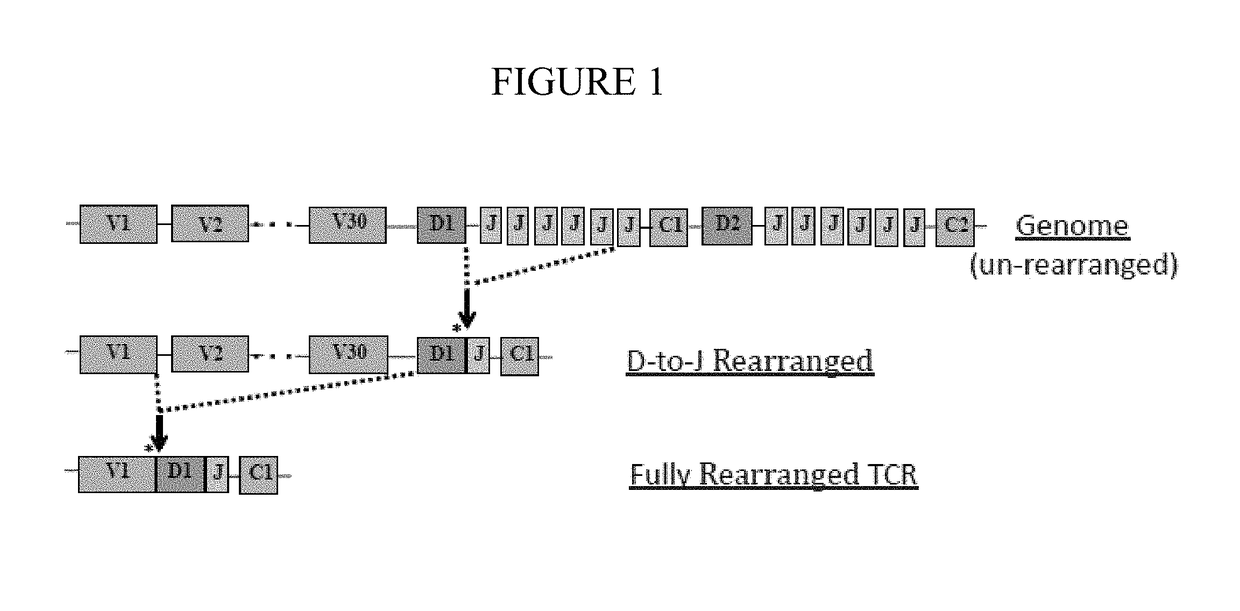
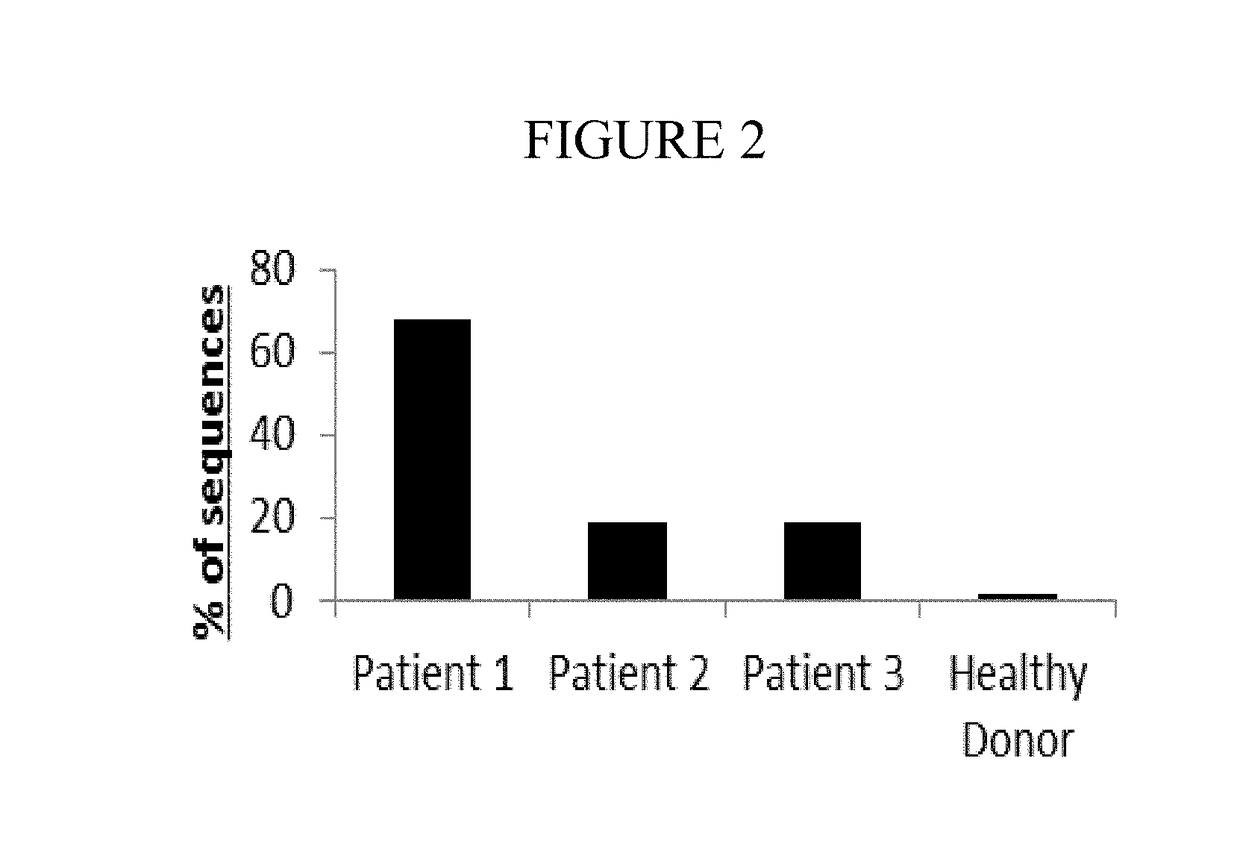
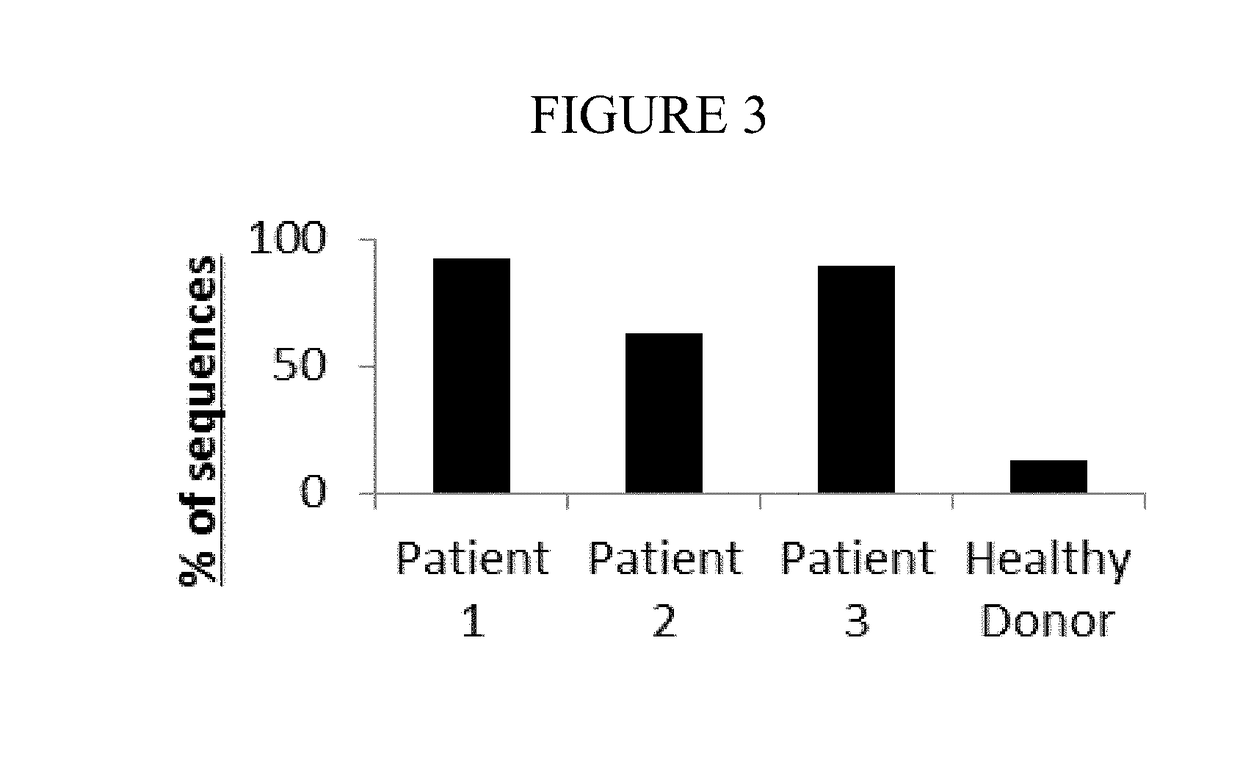
Diagnostic methods for identifying t-cell lymphoma and leukemia by high-throughput tcr-beta sequencing
A method for diagnosing a lymphoid proliferative disorder (such as lymphoma, T-cell lymphoma or leukemia) in a subject or determining the risk of a subject for a lymphoid proliferative disorder recurrence after transient remission, which includes high throughput T-Cell Receptor β (TCRβ) sequencing tests to identify dominant oncogenic clones in the subject suffering from the lymphoid proliferative disorder. Oligonucleotides primer compositions and kits associated with the above-described methods are also provided herein. Said subject includes living organisms, such as mammals (e.g., dogs, cats, pigs, cows, horses, goats, rabbits, humans), non-mammalian vertebrates, such as birds (e.g., chicken, ducks), fish (e.g., sharks), or frogs, and transgenic species thereof.
Owner:KARKINOS PRECISION ONCOLOGY LLC
Glycyrrhetinic acid solid lipid nanoparticles and preparation method for same
InactiveCN102512369AUniform particle sizeHigh encapsulation efficiencyOrganic active ingredientsDigestive systemDiseaseActive agent
The invention relates to glycyrrhetinic acid solid lipid nanoparticles and a preparation method for the same, belonging to the field of medicinal preparation. The main ingredients of the glycyrrhetinic acid solid lipid nanoparticles disclosed by the invention comprise active raw material glycyrrhetinic acid, medicinal phospholipid, a lipid material and a surfactant. The glycyrrhetinic acid solid lipid nanoparticle solution and the freeze-dried powder injection thereof prepared by the preparation method disclosed by the invention are small in particle diameter, high in entrapment efficiency, good in stability and capable of being used for a plurality of administration routes such as oral administration and injection administration. The glycyrrhetinic acid solid lipid nanoparticles disclosed by the invention can reduce dosage, enhance curative effect and reduce the toxic and side effects of medicine, as well as are suitable for treating a plurality of diseases such as hepatitis, liver cancer, lung cancer, ovarian cancer, gastritis, gastric cancer, leukaemia and aids.
Owner:WUHAN UNIV
Compositions Comprising Human Embryonic Stem Cells and Their Derivatives, Methods of Use, and Methods of Preparation
Owner:SHROFF GEETA
Anti-Siglec-15 antibodies and uses thereof
ActiveUS9447192B2Reduce in quantityEliminate side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntibodyMyeloid leukaemia
This disclosure relates to anti-Siglec-15 antibodies and uses thereof, in particular in the treatment of leukaemia, such as acute myeloid leukaemia.
Owner:MEDIMMUNE LTD +1
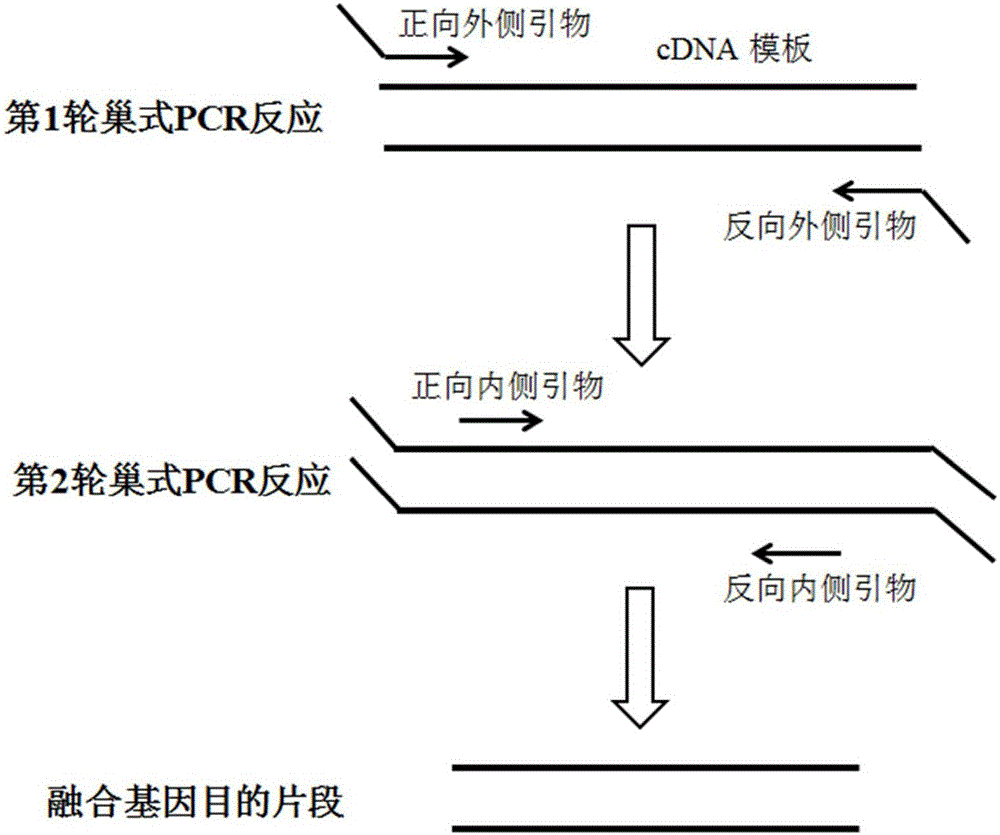
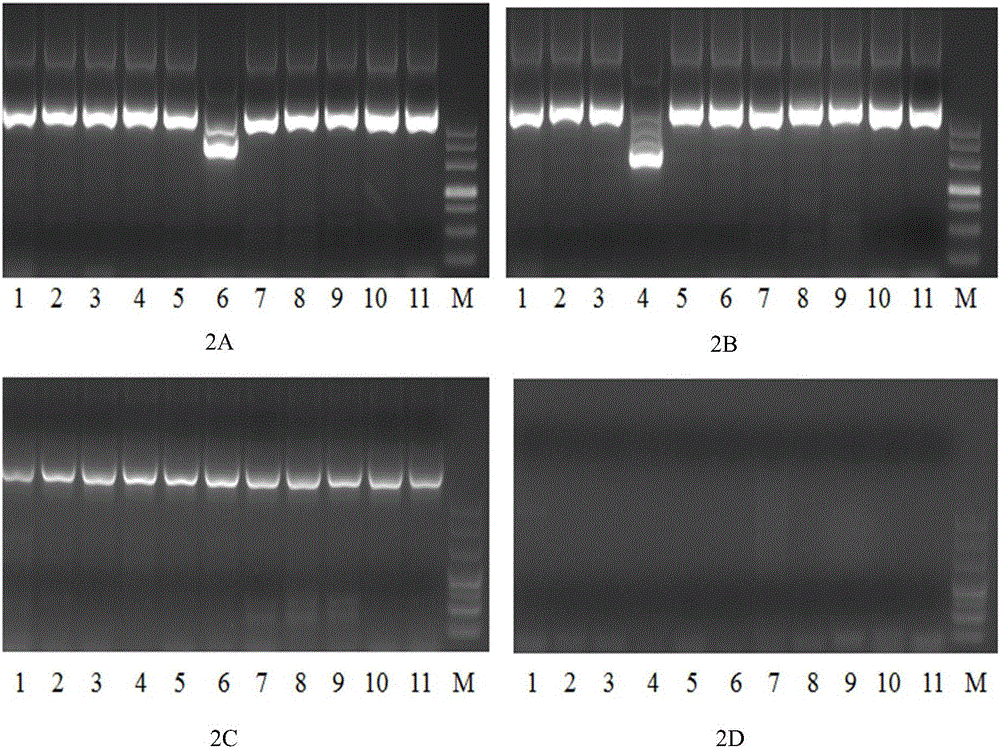
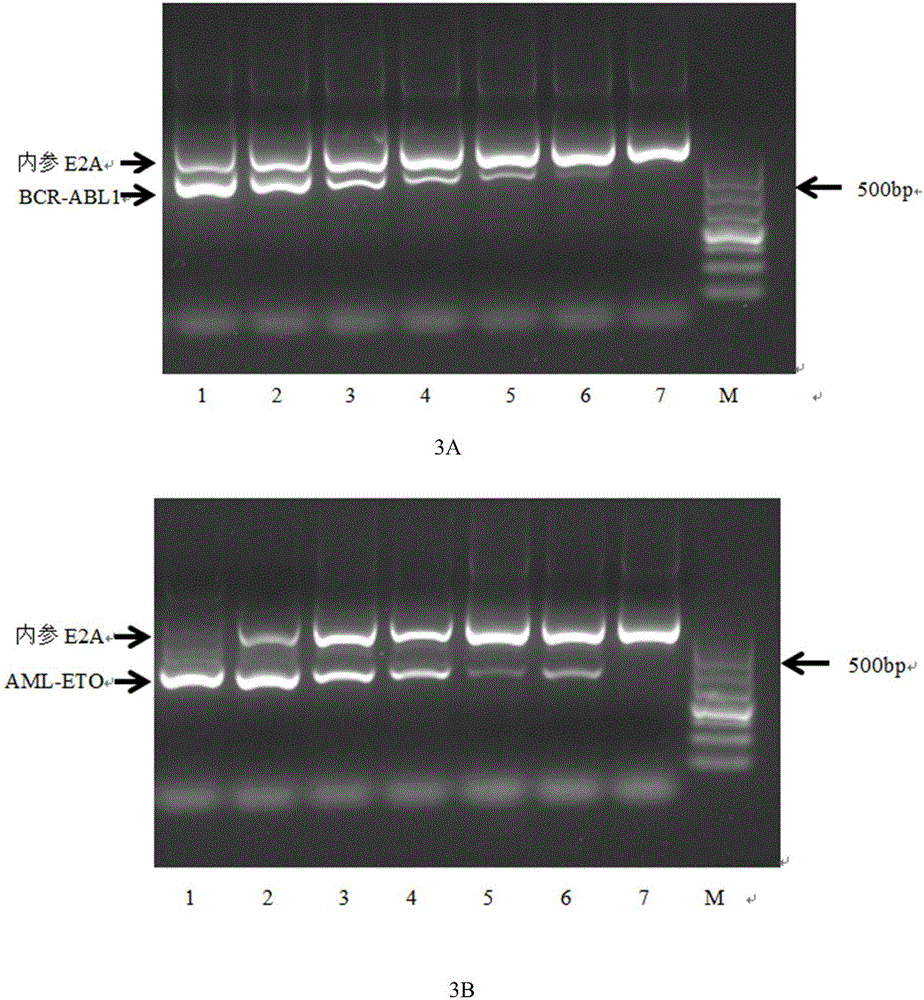
Primers, kit and method for qualitatively detecting leukaemia fusion genes
ActiveCN105838793AImprove featuresImprove efficiencyMicrobiological testing/measurementDNA/RNA fragmentationPcr methodReverse transcription polymerase chain reaction
The invention belongs to the technical field of gene engineering, and discloses a primer combination for detecting leukaemia fusion genes, a kit containing the primer combination, and a multiplex nested RT-PCR (reverse transcription-polymerase chain reaction) method for performing leukaemia fusion gene detection by using the primer combination or kit. The method is based on a multiplex nested RT-PCR technique, and thus, is simple and quick and has high sensitivity. Besides, the reasonable primer combination is utilized to effectively avoid interactions among multiple primer pairs, thereby reducing the detection errors. The detection method can be utilized to comprehensively perform qualitative detection on 43 leukaemia fusion genes, thereby saving the reagent consumption and lowering the detection cost. The detection method has wide detection range, and is suitable for detecting mass samples in clinic.
Owner:SHANGHAI TISSUEBANK BIOTECH +3
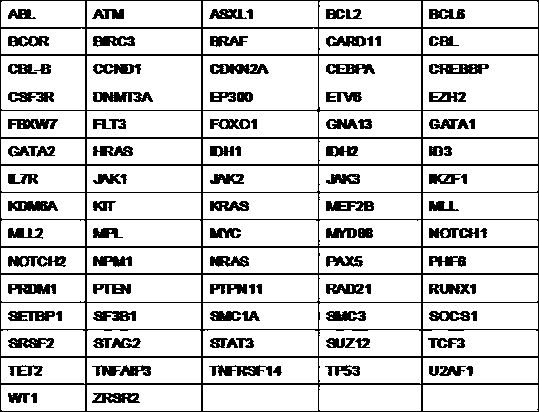
High-throughput assay method for leukaemia driver genes
PendingCN107723353AGuaranteed catchHigh sensitivityMicrobiological testing/measurementAssayIndividualized treatment
The invention discloses a high-throughput assay method for leukaemia driver genes. By choosing mutant genes and fusion genes commonly seen in leukaemia at present, in combination with the second-generation high-throughput sequencing technology, the method can more comprehensively assay the gene mutation and fusion conditions of leukaemia. Compared with the classical Sanger sequencing method mostlyadopted at present, the second-generation high-throughput sequencing technology has the advantages of high throughput, short sequencing time, high precision, low cost and rich information, and can accurately locate and analyze target gents in a short time. With the help of the high-throughput assay technology, the method can provide more detailed gene mutation and fusion spectra for clinical doctors to comprehensively judge the prognosis of leukaemia and guide individualized treatment.
Owner:JIAXING YUNYING MEDICAL INSPECTION CO LTD
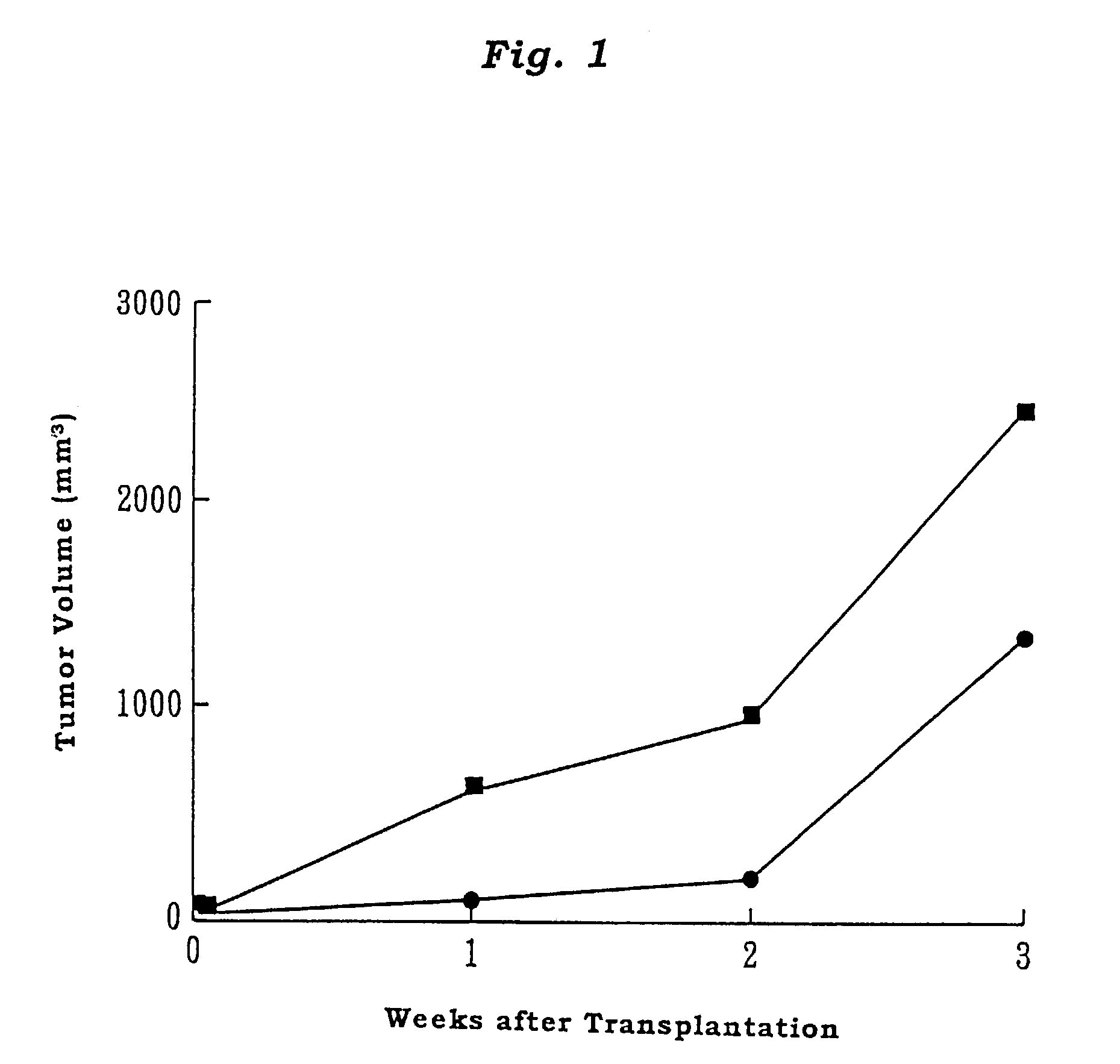
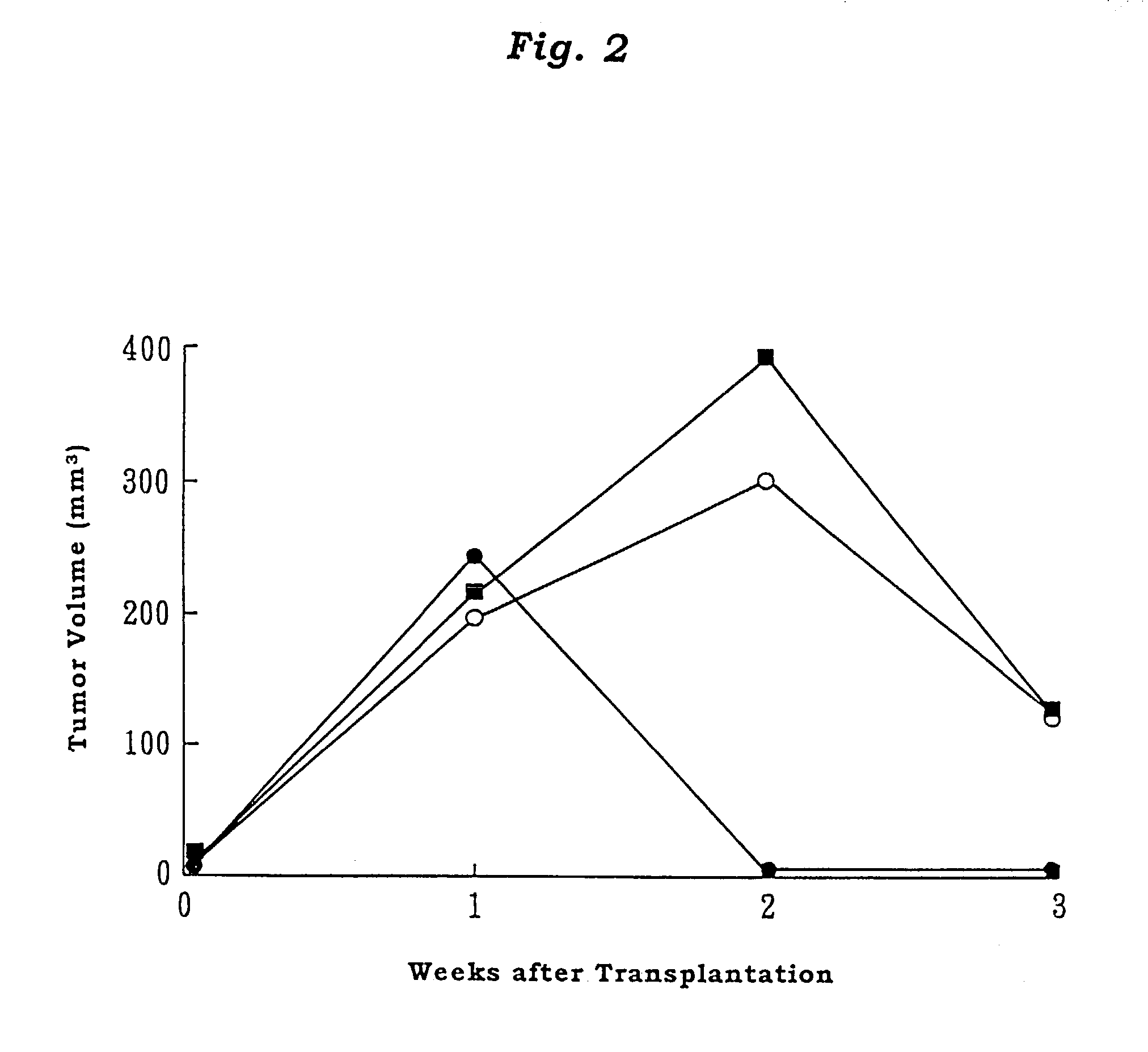
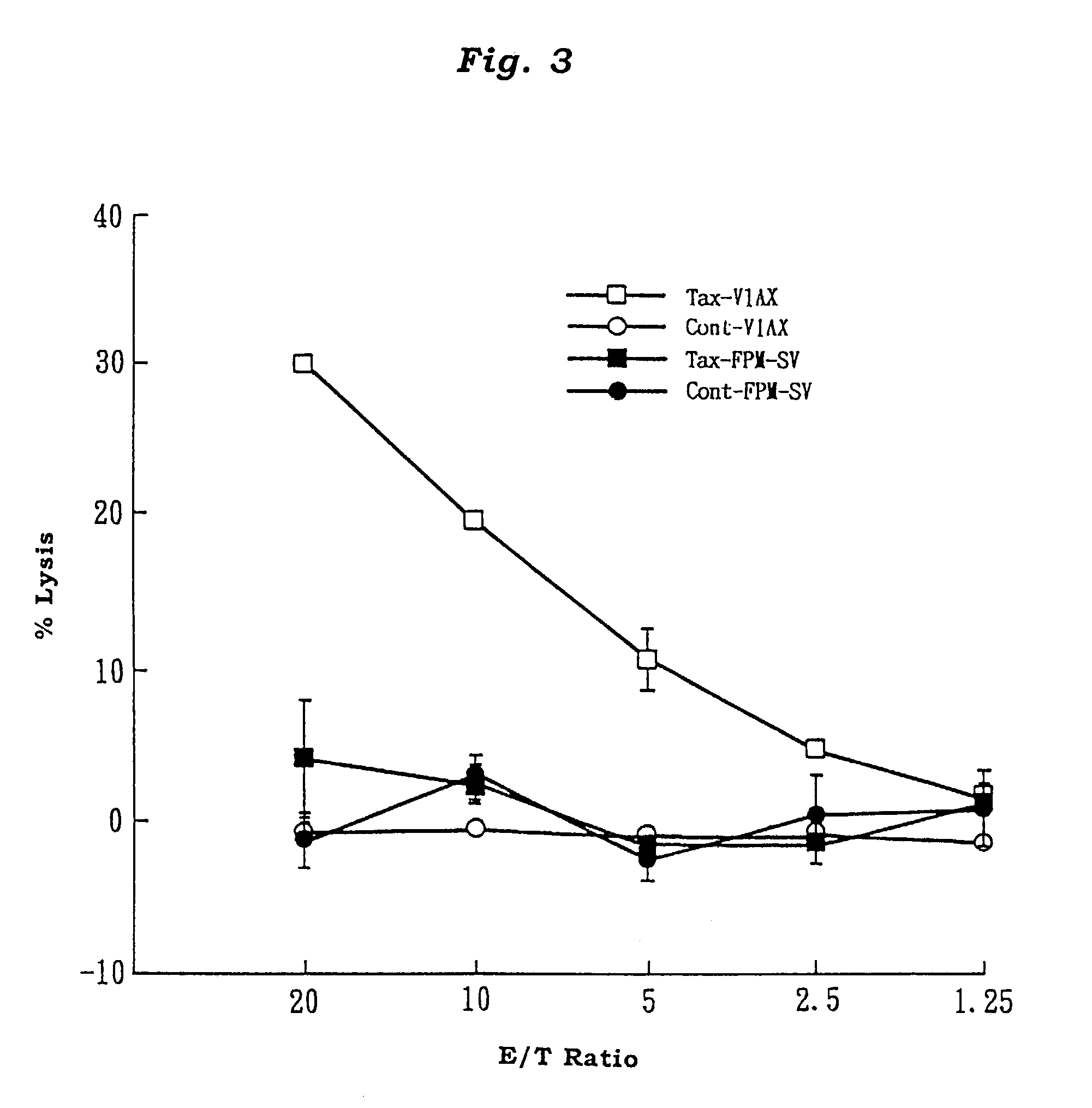
Adult T cell leukemia model animal
InactiveUS7186880B2Blood/immune system cellsTumor/cancer cellsInfected cellHuman T cell leukemia virus
The present invention provides an adult T cell leukemia model animal, which is a T cell function deficient animal to which a cell line infected with human T cell leukemia virus-1 is transplanted. This model animal allows the HTLV-1 infected cell line to proliferate over a long period of time, and enables not only the tumorigenesis process but also the mechanism of onset of ATL and immune response mechanism of the host against ATL to be precisely analyzed.
Owner:JAPAN SCI & TECH CORP
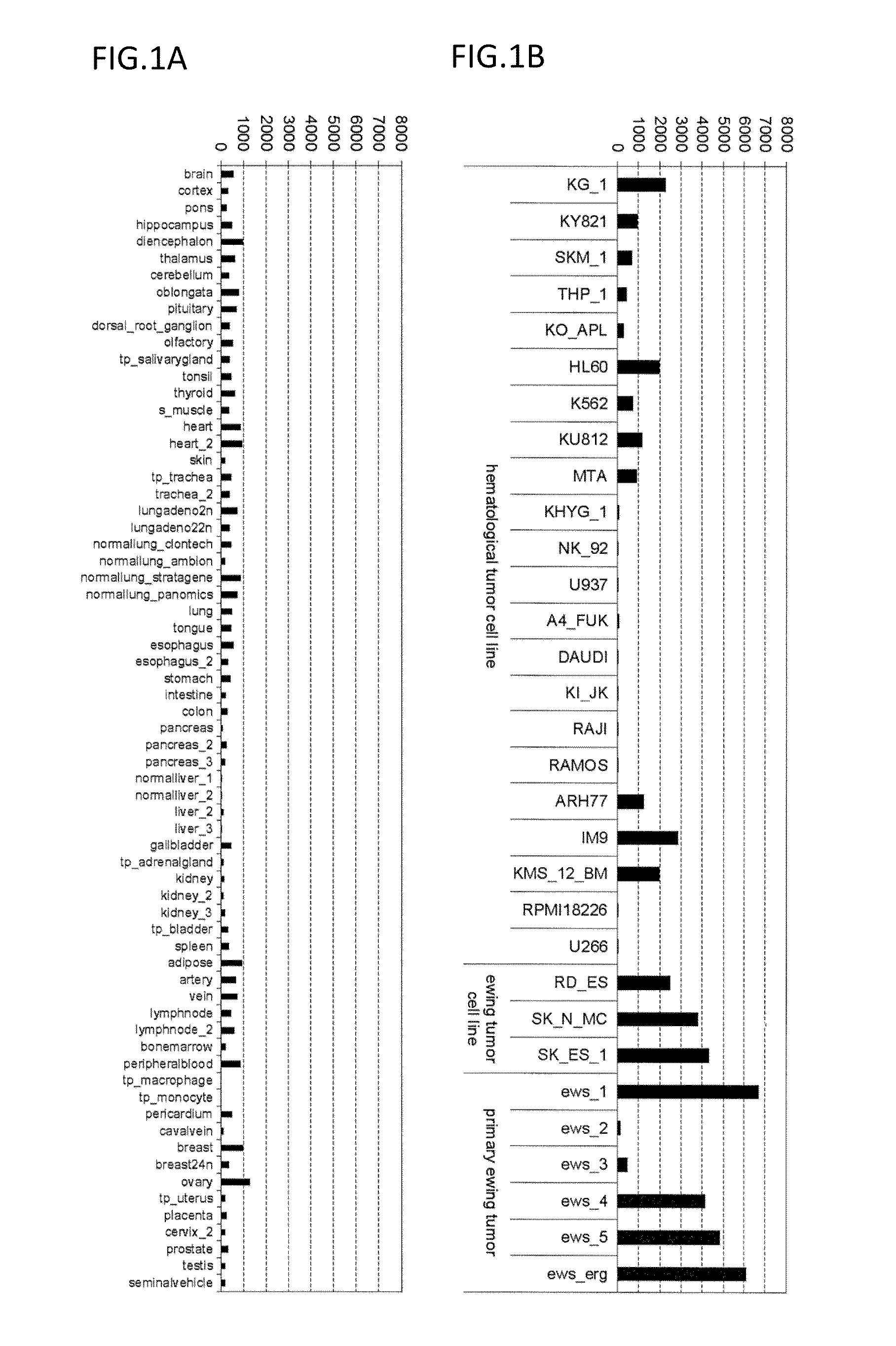
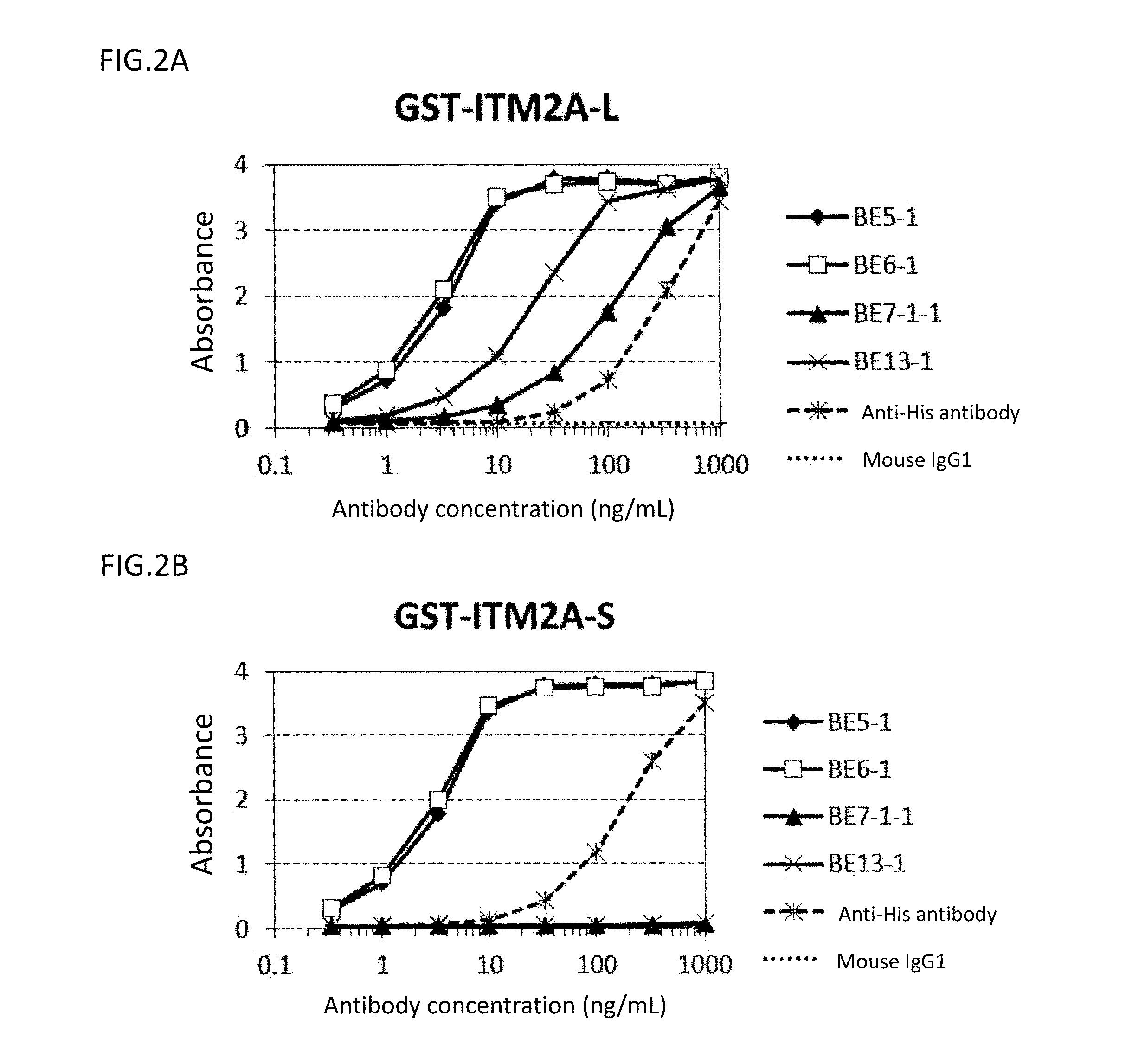
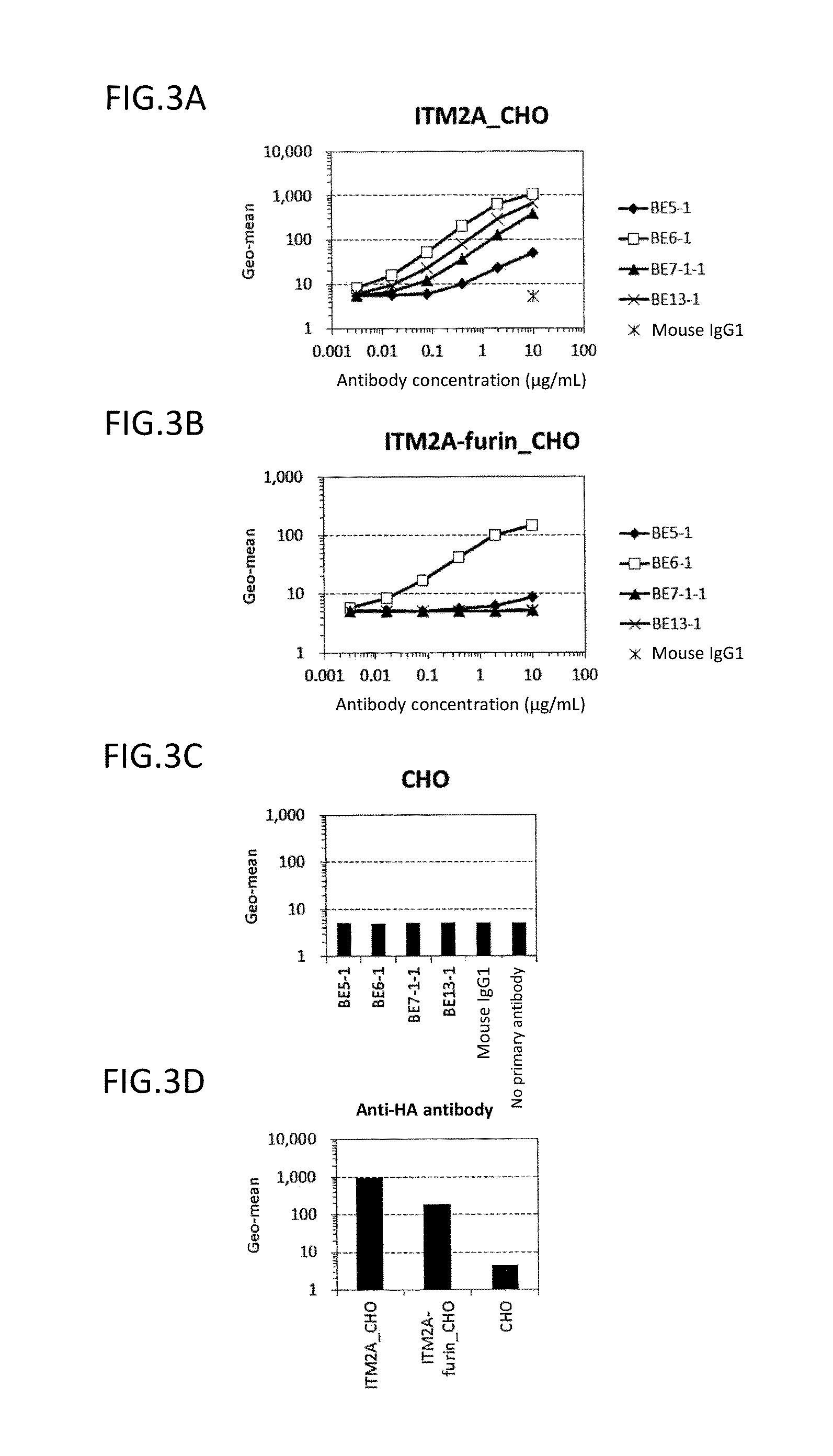
Diagnosis and treatment of cancer using Anti-itm2a antibody
InactiveUS20140193420A1Immunoglobulins against cell receptors/antigens/surface-determinantsDisease diagnosisAnticarcinogenAntiendomysial antibodies
Disclosed is a monoclonal antibody binding to an ITM2A protein. This antibody is useful in the diagnosis, prevention, and treatment of cancer such as Ewing's sarcoma, T cell leukemia, T cell lymphoma, acute myeloid leukemia, B cell tumor, and multiple myeloma. The present invention also provides a pharmaceutical composition, a cell growth inhibitor, and an anticancer agent containing the antibody as an active ingredient, and a method for treating cancer, a method for predicting the efficacy of cancer treatment, and a method for determining the presence of cancer in a test subject using the antibody.
Owner:THE UNIV OF TOKYO +1
Diterpenoid compounds, and preparation method and application thereof
InactiveCN105175252AEnhanced inhibitory effectHigh anticancer activityAntineoplastic agentsCarboxylic compound separation/purificationChromatographic separationStructural formula
The invention discloses diterpenoid compounds, and a preparation method and application thereof. The diterpenoid compounds are prepared by the following steps: by using Aralia melanocarpa root as a raw material, carrying out extract leaching, organic solvent extraction, silica gel column chromatography and high pressure liquid chromatography separation. The molecular formula of the compounds is C20H28O2 which is named ent-pimar-6,8(14),15-trien-19-oic acid disclosed as the structural formula in the specification. The preparation method comprises the following steps: by using the Aralia melanocarpa root as the raw material, carrying out extract leaching, organic solvent extraction, silica gel column chromatography and high pressure liquid chromatography separation. The invention also discloses application of the diterpenoid compounds in preparing drugs for preventing and / or treating tumor and in preparing drugs for preventing and / or treating human lung adenocarcinoma, human prostatic cancer or human acute medullary system leukaemia. The diterpenoid compounds have obvious inhibiting actions when being applied to drugs for human lung adenocarcinoma, human prostatic cancer or human acute medullary system leukaemia, which indicates that the diterpenoid compounds have favorable anticancer activity and can be used as an anticancer active component or lead compound.
Owner:YUNNAN MINZU UNIV
Anti-tumor active matter and its preparing method and use
InactiveCN1682771AHas antitumor activityAchieve production controlFungiBacteria material medical ingredientsActive matterTyrosine
The present invention relates to active antitumor matter and its preparation process and use, and belongs to the field of microbiological medicine technology. The active matter is prepared through convenient process with Moinascus ruber 3081 of preservation number CGMCC No. 1319. The test seed is cultured in conventional malt juice culture medijm at 20-30 deg.c for 4-8 days; and the solid fermentation culture medium consists of notoginseng hairy rootlet powder 20-30 wt% and water 70-80 wt% in natural pH. Through further solid culture at 20-30 deg.c for 15-20 days, alcohol leaching with alcohol solution for 2 days, decompression concentrating and evaporating out solvent, the active matter is obtained. The active matter has high inhibiting rate to human leukaemia cell strain K562, human lung cancer strain A549, oncogene Raf1 and c-Myc latent target tyrosine phosphoesterase cdc25a and cdc25b, so that it may be used in preparing antitumor preparation.
Owner:YUNNAN UNIV
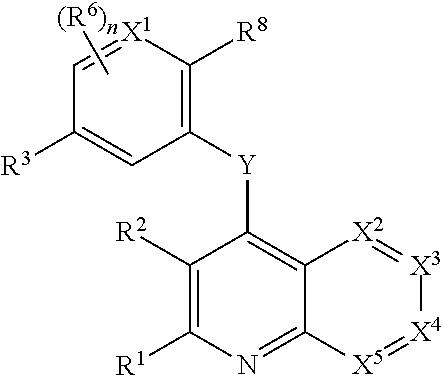
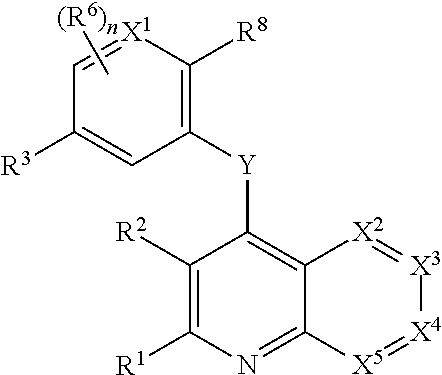
Heterocyclic compounds and their uses
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110 activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com