Cd123-specific chimeric antigen receptor redirected t cells and methods of their use
A technology of chimeric antigen receptors and cells, applied in the direction of receptors/cell surface antigens/cell surface determinants, antibodies, animal cells, etc., can solve problems such as recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
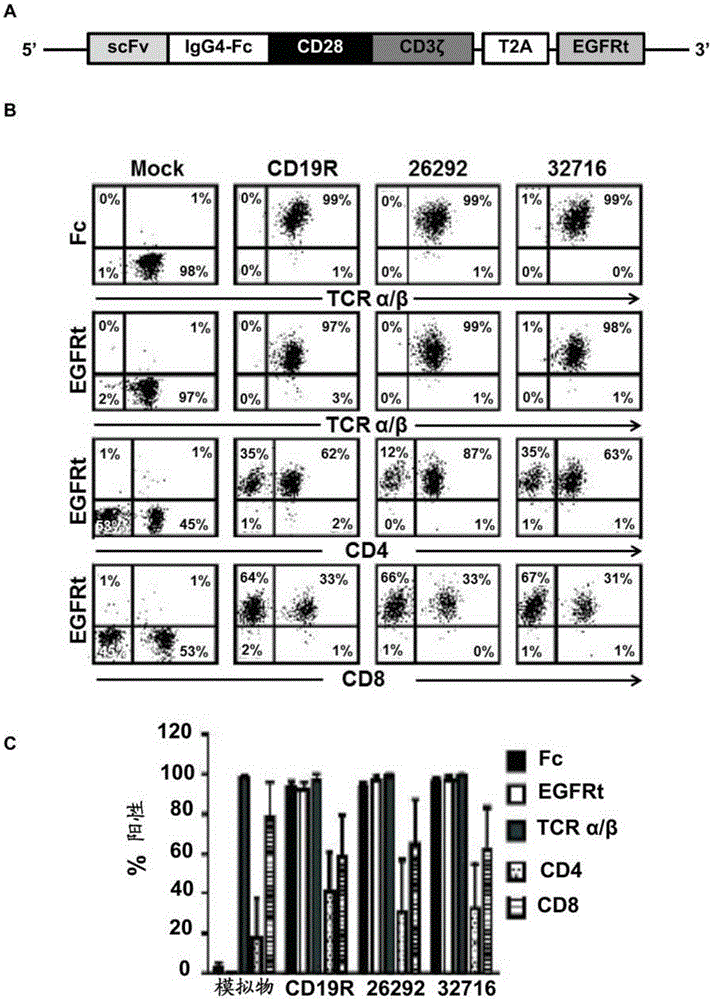
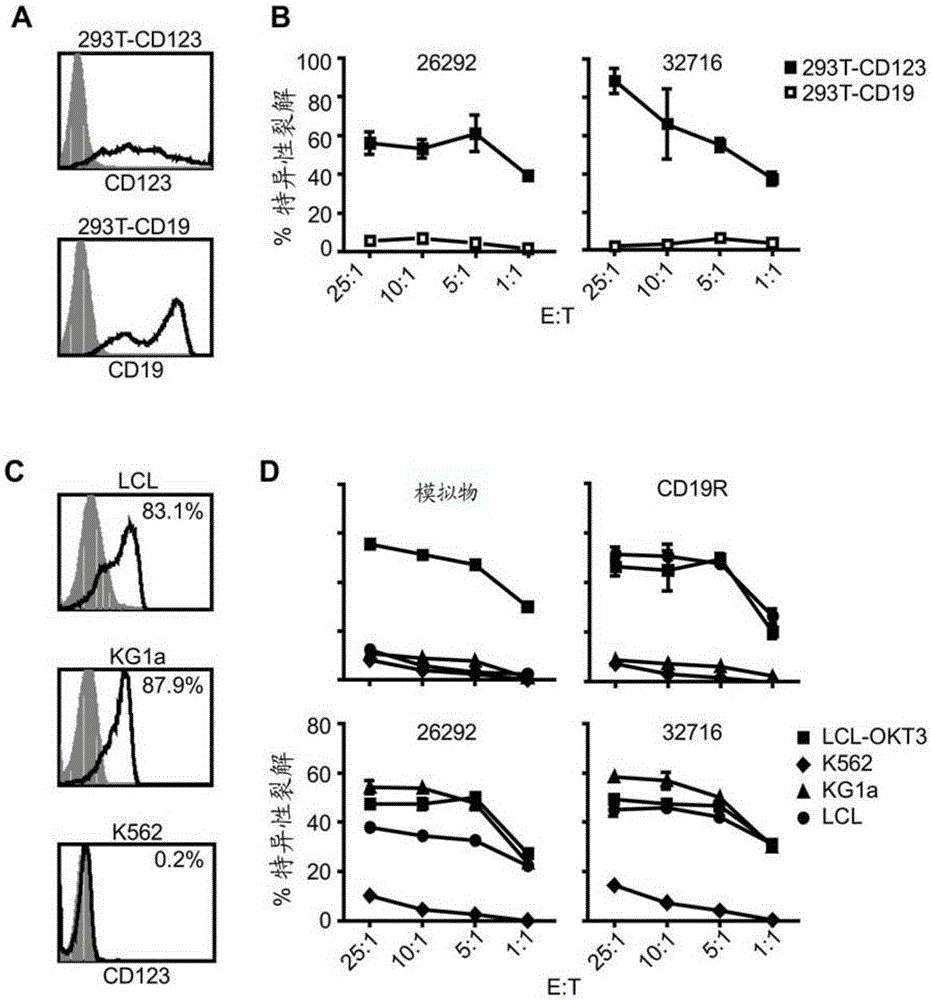
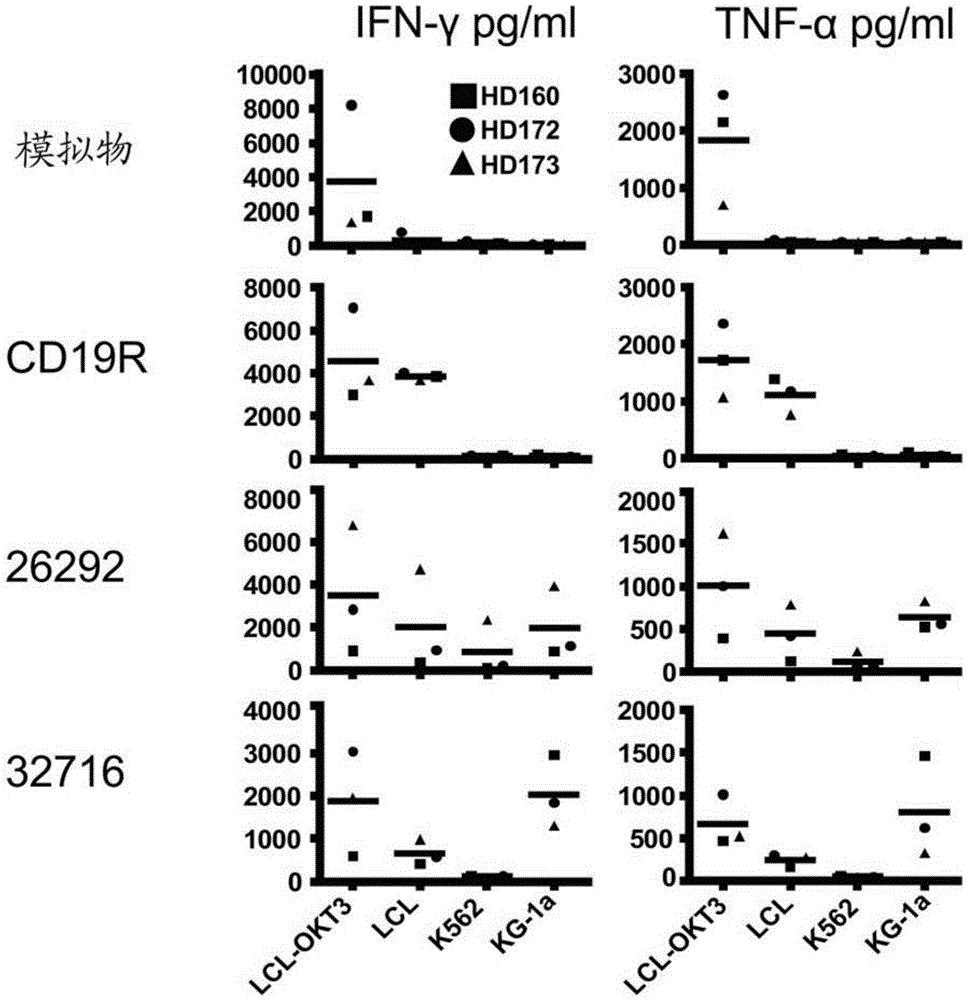
[0104] Example 1: T cells transduced with CD123CAR exhibit potent cytolytic activity and multiple effector functions against AML in vitro
[0105] Materials and methods
[0106] Cell lines. Unless otherwise stated, all cell lines were maintained in RPMI 1640 (Irvine Scientific) supplemented with 2 mM L-glutamine, 25 mM HEPES, and 10% heat-inactivated FCS (Hyclone) (hereinafter referred to as complete medium (CM)). Peripheral blood mononuclear cells (PBMC) were transformed with Epstein-Barr virus to generate lymphoblastoid cell lines (LCL) as previously described [19]. LCL-OKT3 cells express membrane-bound OKT3 and were grown in CM supplemented with 0.4 mg / ml hygromycin [20]. K562 cells were obtained from ATCC and cultured as recommended. KG1a cells (kindly provided by Dr. Ravi Bhatia) were maintained in IMDM (Irvine Scientific) supplemented with 25 mM HEPES, 4 mM L-glutamine (Irvine Scientific) and 20% FCS. 293T cells (a kind gift from the Center for Biomedicine and Genetic...
Embodiment 2
[0149] Example 2: T cells transduced with CD123CAR delay leukemia progression in vivo
[0150] CD123CAR constructs. The 26292CAR (S228P+L235E) and 32716CAR (S228P+L235E) constructs were generated as described in Example 1 above. Two additional CD123CAR constructs containing an additional mutation (N297Q) at position 297 in the IgG4 hinge for each scFv (“26292CAR(S228P+L235E+N297Q)” and “32716CAR(S228P+L235E+N297Q)” were also generated N297Q)")( Figure 12 and 13 , mutate bold and underline).
[0151] NSG mice were transplanted with AML tumor cells (day 0) and treated with 5.0x10 6 CAR+ T cells were processed and leukemia progression was monitored by bioluminescent imaging. Such as Figure 8 Shown in , the leukemic burden progressed at day 8 compared to the treatment day for mice treated with T cells transduced with 26292CAR(S228P+L235E), indicative of the use of the CD123CAR construct with hinge region mutations at positions S228P and L235E Transduced cells have no effec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com