Patents
Literature
97 results about "Biological immortality" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
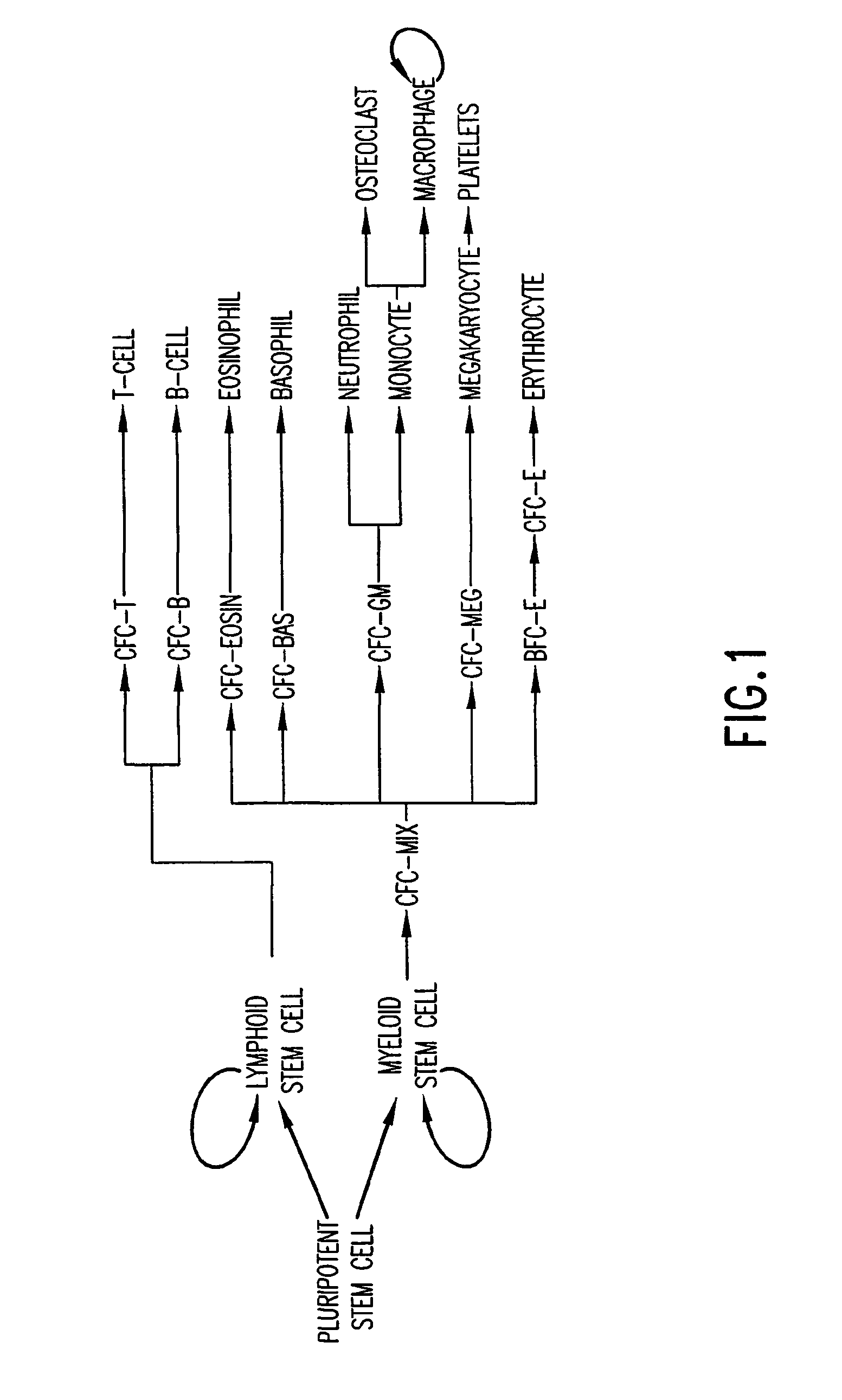
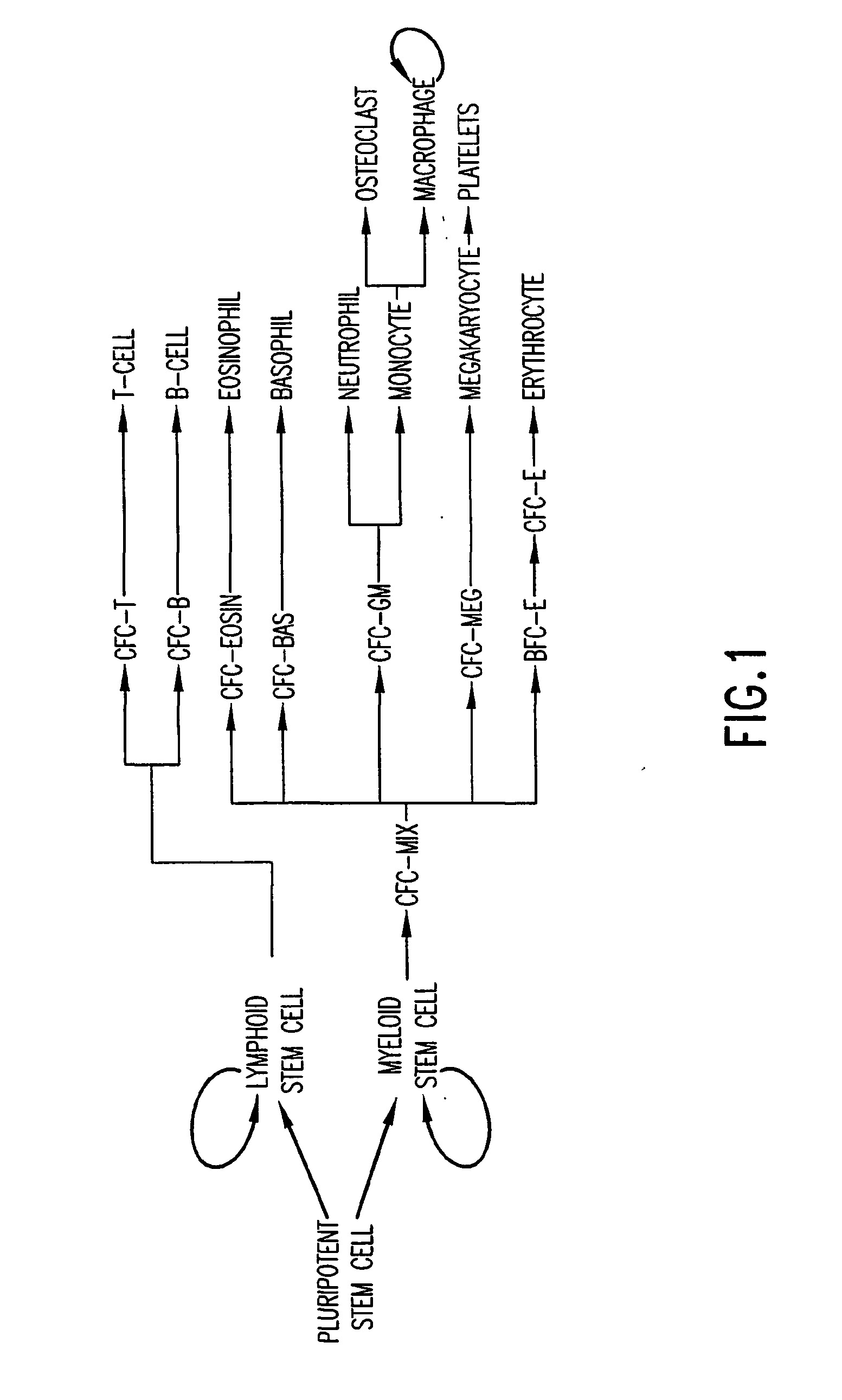
Biological immortality (sometimes referred to as bio-indefinite mortality) is a state in which the rate of mortality from senescence is stable or decreasing, thus decoupling it from chronological age. Various unicellular and multicellular species, including some vertebrates, achieve this state either throughout their existence or after living long enough. A biologically immortal living being can still die from means other than senescence, such as through injury, disease, or lack of available resources.
Methods for immortalizing cells
InactiveUS7399633B2Antibody mimetics/scaffoldsGenetically modified cellsCell Differentiation processCultured cell
The present invention provides methods for immortalizing precursor cells that are non-terminally differentiated cells such as stem cells, said methods comprising culturing the precursor cells in the presence of a Notch agonist and one or more growth factors that support the proliferation but not differentiation of the non-terminally differentiated cells. The present invention further provides methods to induce the differentiation of immortalized cells, comprising growing the cells in the presence of a Notch agonist and at least one growth factor which supports the differentiation of the cell into a more specialized cell type. The immortalized and / or differentiated cells of the invention can be used to repopulate cell populations that have been diminished, for example as a result of infection or exposure to certain drugs. The invention further provides a cell culture comprising a population of non-terminally differentiated cells immortalized by the methods of the present invention and kits comprising reagents that promote the immortalization of precursor cells. The invention further provides methods for screening for Notch modulators and for identifying genes involved in processes of cellular differentiation.
Owner:FRED HUTCHINSON CANCER CENT
Methods for immortalizing cells
InactiveUS20040067583A1Antibody mimetics/scaffoldsGenetically modified cellsCultured cellSomatic cell
The present invention provides methods for immortalizing precursor cells that are non-terminally differentiated cells such as stem cells, said methods comprising culturing the precursor cells in the presence of a Notch agonist and one or more growth factors that support the proliferation but not differentiation of the non-terminally differentiated cells. The present invention further provides methods to induce the differentiation of immortalized cells, comprising growing the cells in the presence of a Notch agonist and at least one growth factor which supports the differentiation of the cell into a more specialized cell type. The immortalized and / or differentiated cells of the invention can be used to repopulate cell populations that have been diminished, for example as a result of infection or exposure to certain drugs. The invention further provides a cell culture comprising a population of non-terminaly differentiated cells immortalized by the methods of the present invention and kits comprising reagents that promote the immortalization of precursor cells. The invention further provides methods for screening for Notch modulators and for identifying genes involved in processes of cellular differentiation.
Owner:FRED HUTCHINSON CANCER CENT
Indefinite culture of human adult glia without immortalization and therapeutic uses thereof
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Immortalized canine adipic mesenchymal stem cell line and constructing method thereof
InactiveCN105779395APromote growthMultidirectional differentiation potential performanceMicroorganism based processesSkeletal/connective tissue cellsStem cell lineMesenchymal stem cell
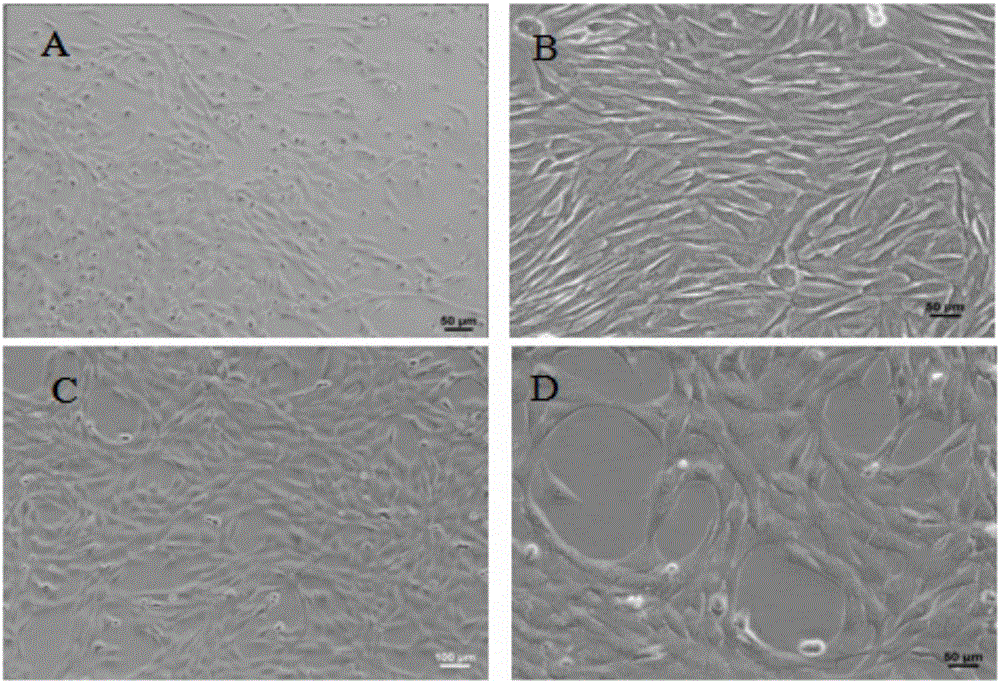
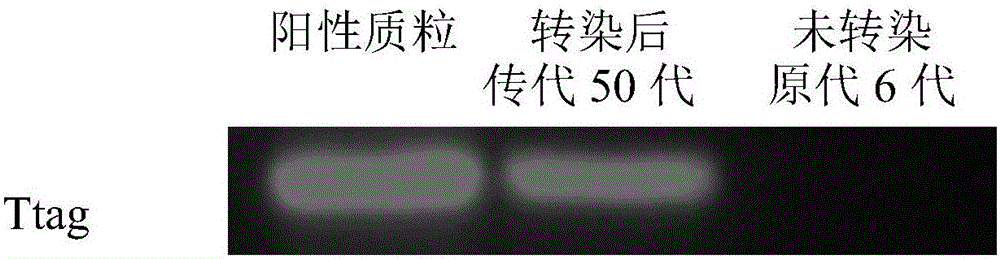
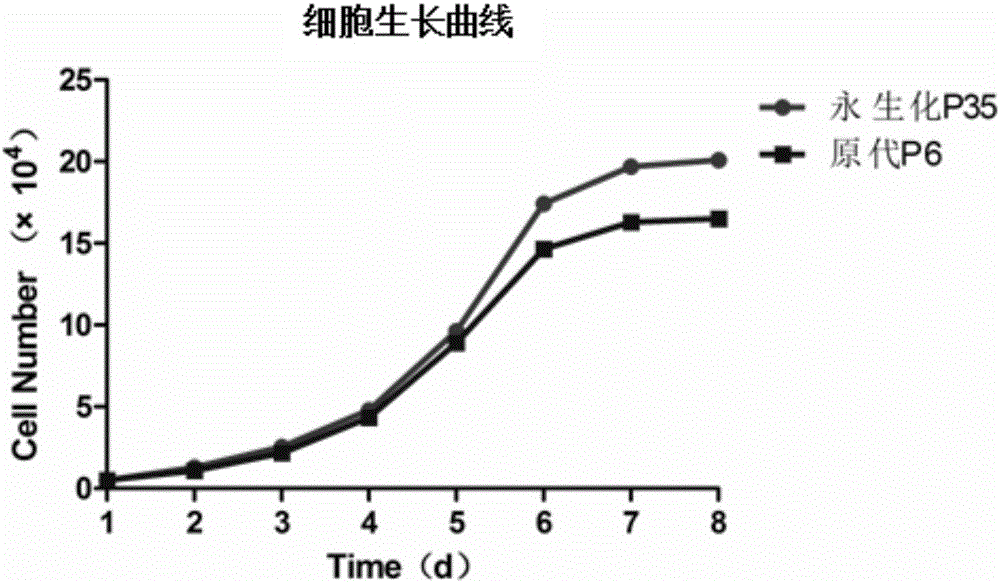
The invention discloses an immortalized canine adipic mesenchymal stem cell line and a constructing method thereof. The immortalized canine adipic mesenchymal stem cell line capable of expressing SV40 large T antigen genes and having multidirectional differentiation is obtained by: transfecting a lentiviral vector carrying SV40 large T antigen (Ttag) by using canine adipic mesenchymal stem cells as host cells, integrating the Tag genes into an adipic mesenchymal stem cell genome and carrying out continuous passage and screening. This cell line is still active in case of 50 in-vitro passages, is high in differentiation and proliferation performance, never ages or dies, can also save medium cost and is an immortalized cell line.
Owner:NORTHWEST A & F UNIV
Establishment of immortal human ovary carcinoma cell strain
InactiveCN1336431AHigh degree of malignancyFused cellsVector-based foreign material introductionMicroorganismBiological property
The present invention relates to the method of establishing human ovary cancer immortal cell strian, the immortal gene-SV40T antigen gene is introduced into human ovary cancer original generation cell, then screened, resistance clone enlarge culture to obtain immortal cell strain. The established ovary cancer cell strain is named as BVPH:OVSC-2 and is kept by CGMCC with keeping No.0606, its biological characteristics includes strong invading powder, cell is spindle sarcoma call shape, apparent heteromorphism, retains biological characters of original cell.
Owner:PEOPLES HOSPITAL PEKING UNIV
Use of human prostrate cell lines in prostate cancer treatment
InactiveUS6972128B1Promote growthSufficient characteristicBiocidePeptide/protein ingredientsGamma irradiationProstate cancer
The invention here relates to a product comprised of a cell line or lines intended for use as an allogeneic immunotherapy agent for the treatment of cancer in mammals and humans. All of the studies of cell-based cancer vaccines to date have one feature in common, namely the intention to use cells that contain at least some TSAs and / or TAAs that are shared with the antigens present in patients' tumour. In each case, tumour cells are utilised as the starting point on the premise that only tumour cells will contain TSAs or TAAs of relevance, and the tissue origins of the cells are matched to the tumour site in patients. A primary aspect of the invention is the use of immortalised normal, non-malignant cells as the basis of an allogeneic cell cancer vaccine. Normal cells do not possess TSAs or relevant concentrations of TAAs and hence it is surprising that normal cells are effective as anti-cancer vaccines. For prostate cancer, for example, a vaccine may be based on one or a combination of different immortalised normal cell lines derived from the prostate. The cell lines are lethally irradiated utilising gamma irradiation at 50–300 Gy to ensure that they are replication incompetent prior to use in the mammal or human.
Owner:ONYVAX
Methods for culturing human myeloid leukaemia cells and cells derived therefrom
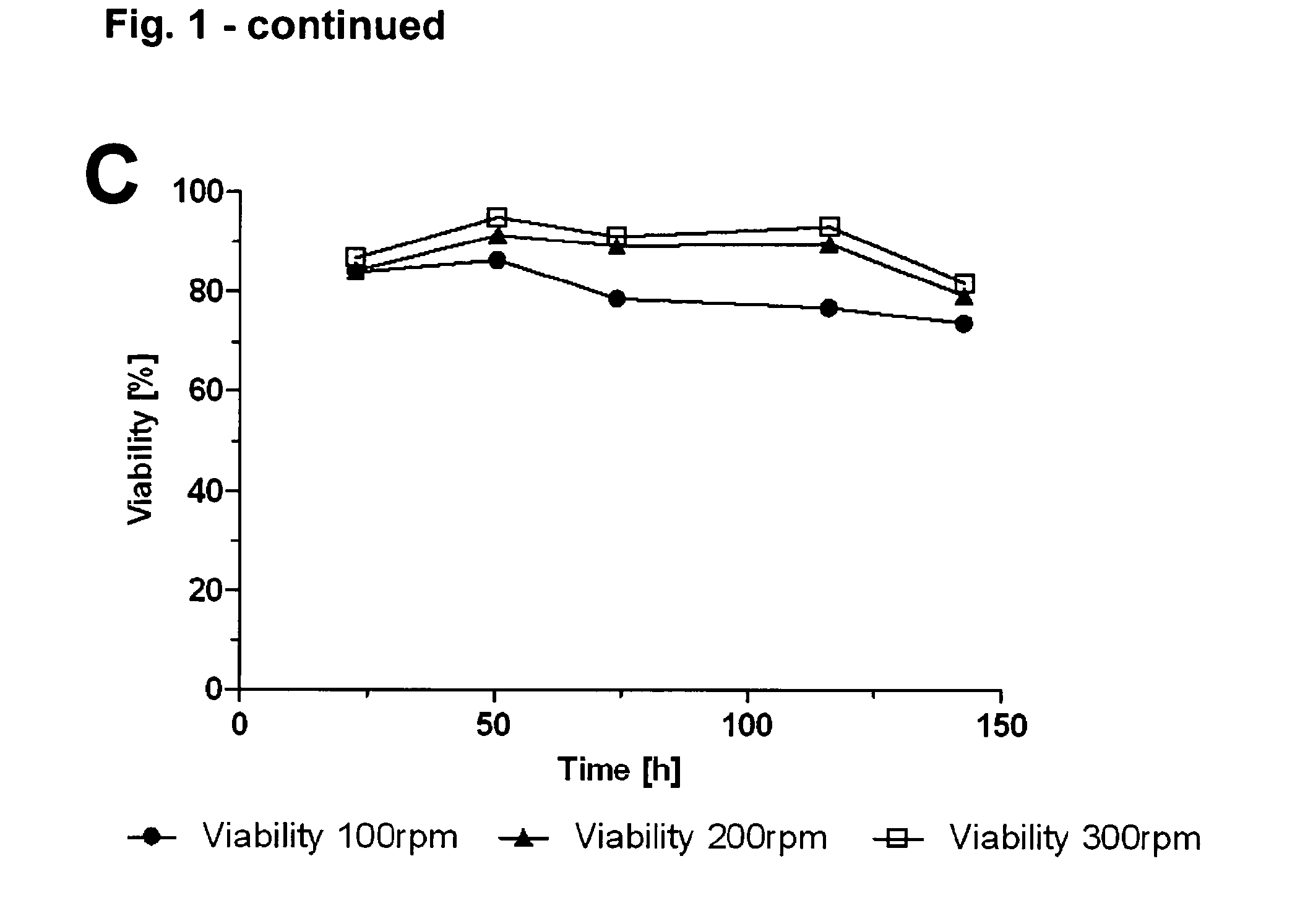
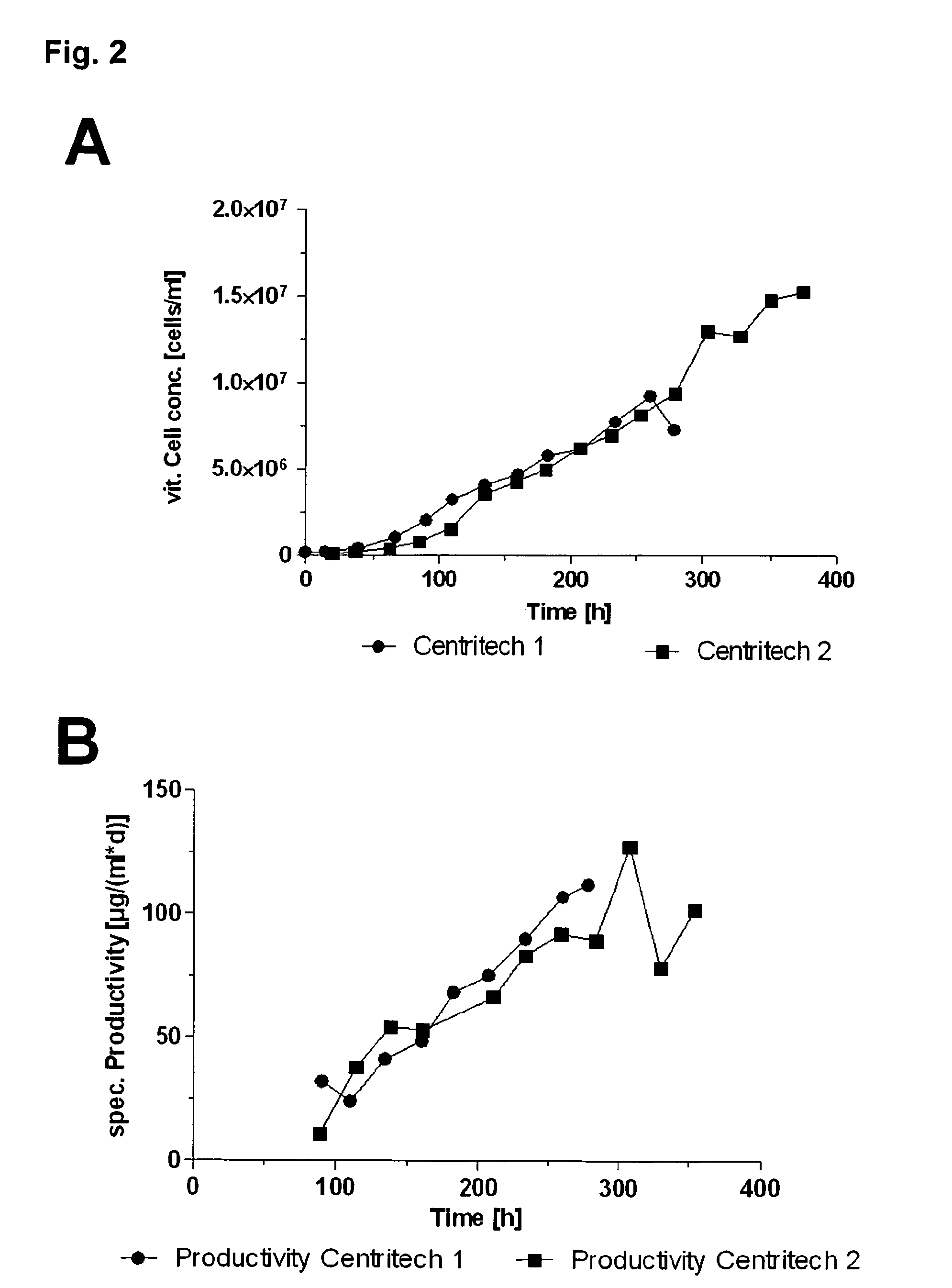
InactiveUS20130330768A1Enhance stirringHigh yieldCulture processArtificial cell constructsBiological immortalityCell biology
The present invention pertains to a method for culturing a suspension of immortalized human blood cells, preferably cells of myeloid leukaemia origin or cells derived therefrom, wherein said method provides a high productivity, a high cell viability and growth rate and a high batch-to-batch consistency, and can be scaled up without altering these parameters.
Owner:GLYCOTOPE GMBH
Use of Anti-Mortalin 2 Antibody and Functional Nucleic Acid for Cancer Therapies
ActiveUS20080260739A1Novel and effective anticancer agentSugar derivativesMicrobiological testing/measurementTumor growthTumor tissue
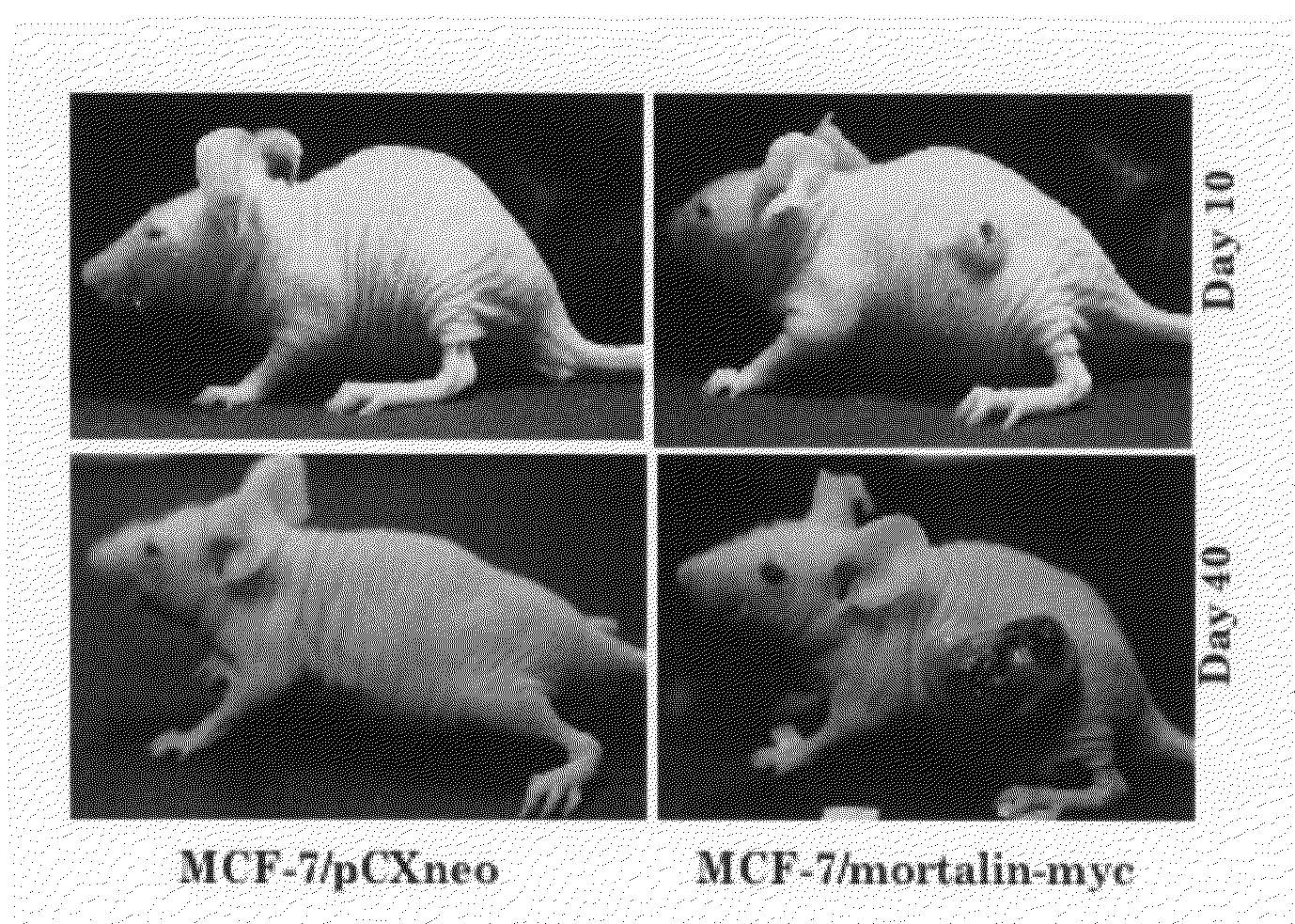
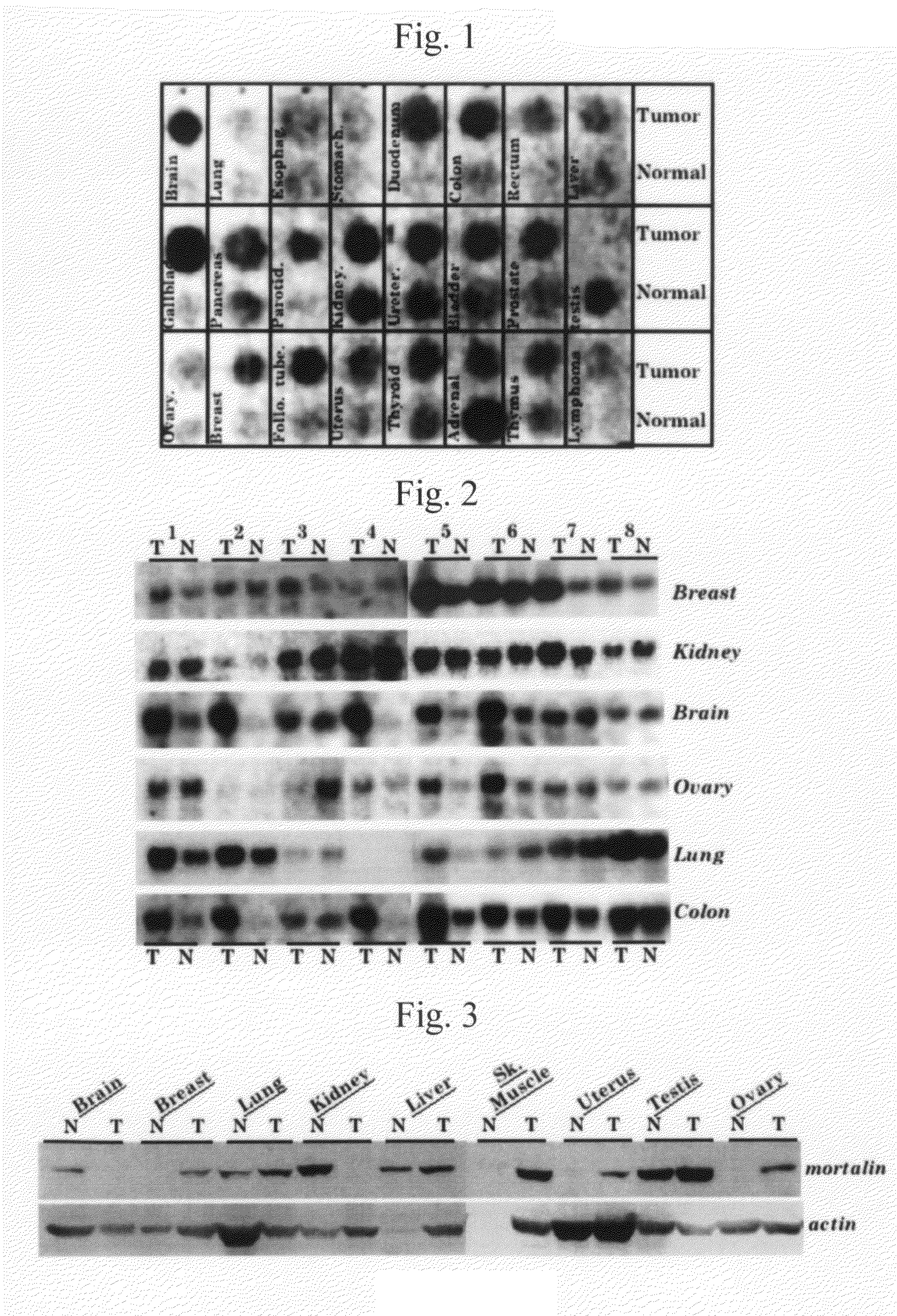
The present invention relates to cancer therapies using an antibody that binds to mortalin 2 and a functional nucleic acid. Mortalin expression was found to be upregulated in immortalized cells and tumor tissues. Immortalized human cells highly expressing mortalin showed anchorage-independent growth. When the K antibody, which is a specific anti-mortalin antibody, was injected into a tumor of a nude mouse, tumor growth was suppressed or the tumor shrank compared with the case of a control. In accordance with the present invention, the use of a specific anti-mortalin antibody (K antibody) for tumor therapies and the use of such antibody as a carrier molecule for transportation of immunotoxicin and the like into cells are provided. It has been shown that mortalin can be a target for cancer therapies. In accordance with the present invention, a novel and effective anticancer agent is provided. In addition, an anti-mortalin antibody that is internalized by cells is developed. Thus, various applications using such antibody are provided.
Owner:NAT INST OF ADVANCED IND SCI & TECH
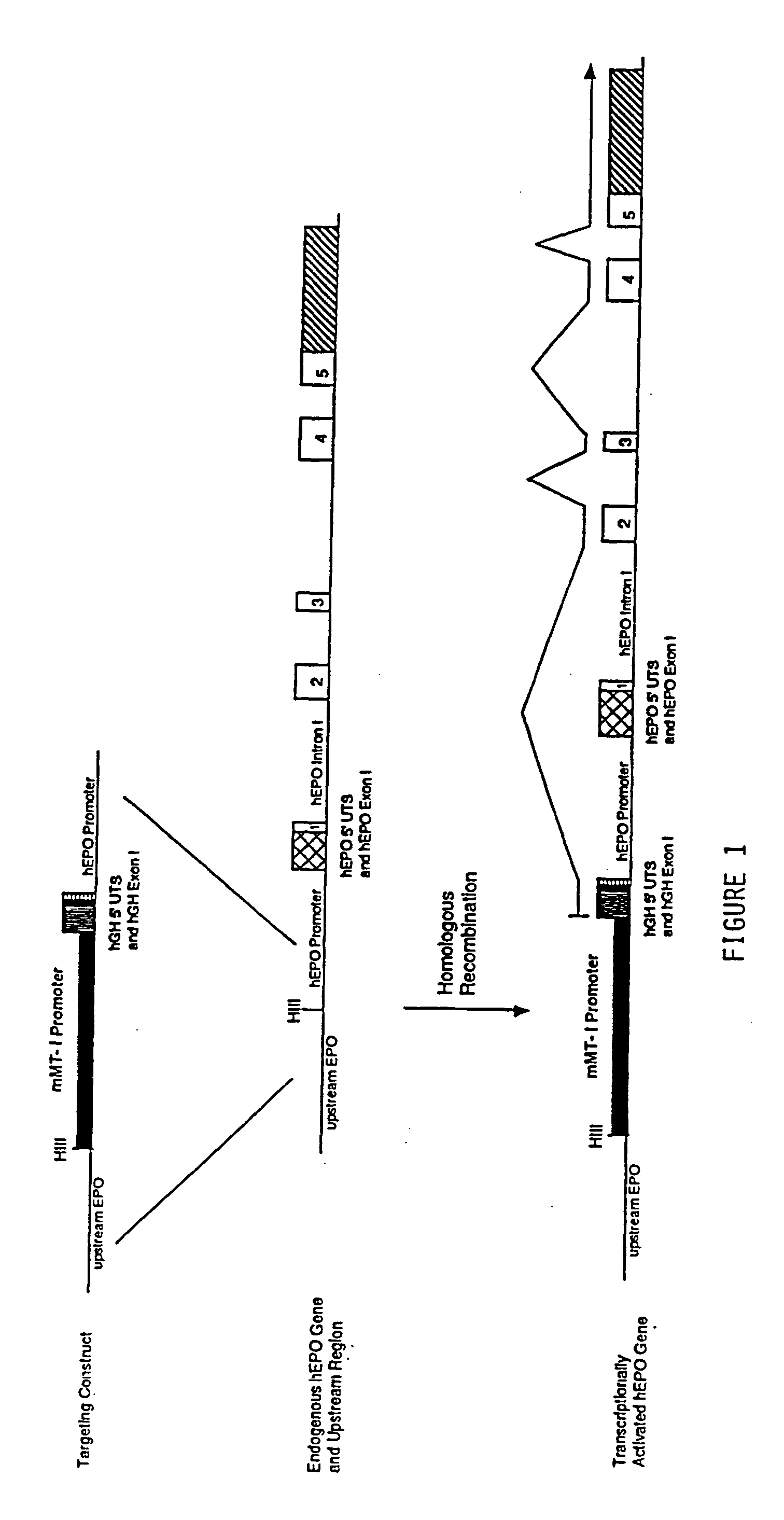
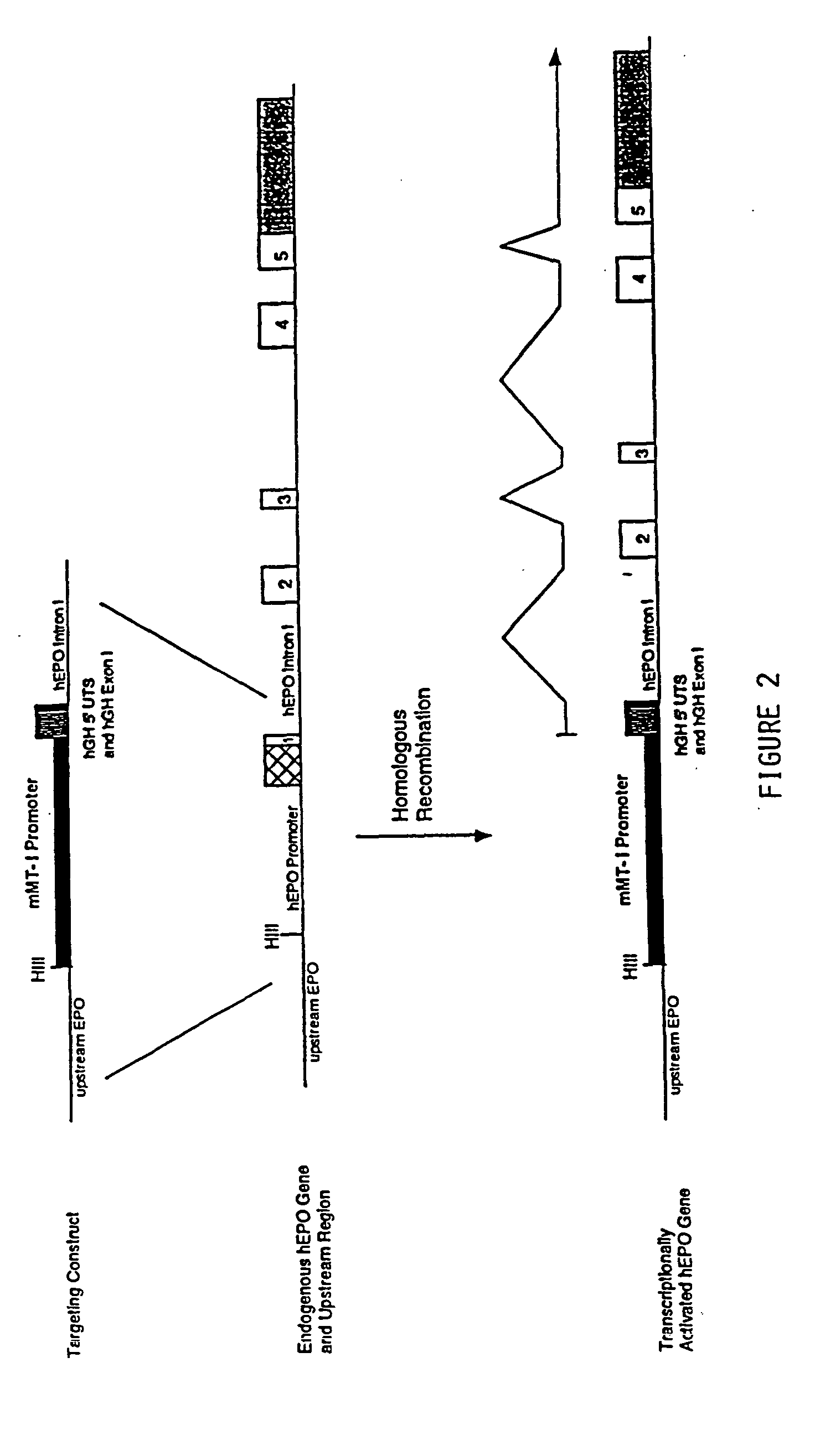
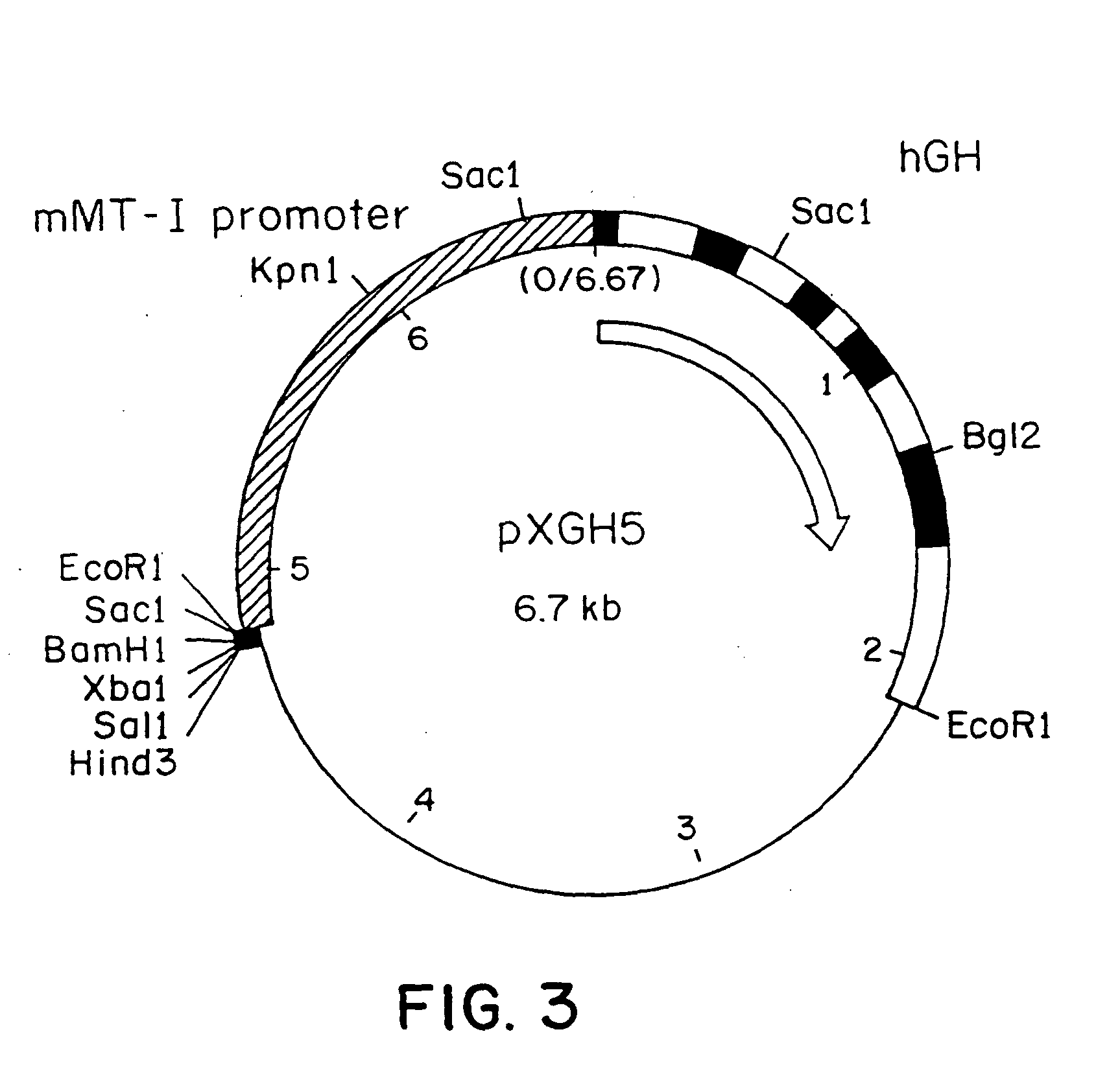
DNA construct for effecting homologous recombination and uses thereof
The invention relates to constructs comprising: a) a targeting sequence; b) a regulatory sequence; c) an exon; and d) an unpaired splice-donor site. The invention further relates to a method of producing protein in vitro or in vivo comprising the homologous recombination of a construct as described above within a cell. The homologously recombinant cell is then maintained under conditions which will permit transcription and translation, resulting in protein expression. The present invention further relates to homologously recombinant cells, including primary, secondary, or immortalized vertebrate cells, methods of making the cells, methods of homologous recombination to produce fusion genes, methods of altering gene expression in the cells, and methods of making a protein in a cell employing the constructs of the invention.
Owner:TRANSKARYOTIC THERAPIES
Senescene Cells and Methods For Its Production
InactiveUS20110189142A1BiocideGenetic material ingredientsPhases of clinical researchSenescence function
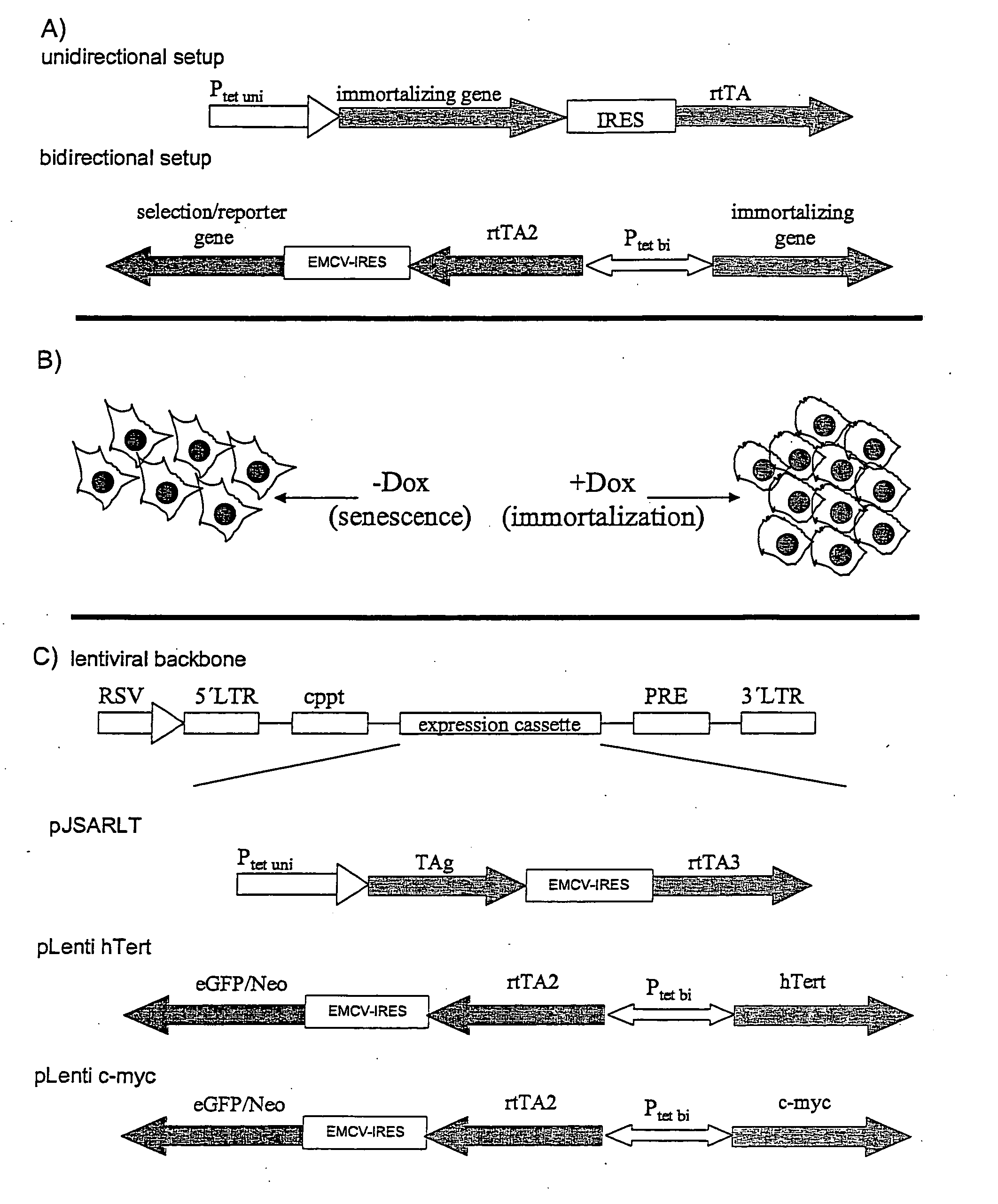
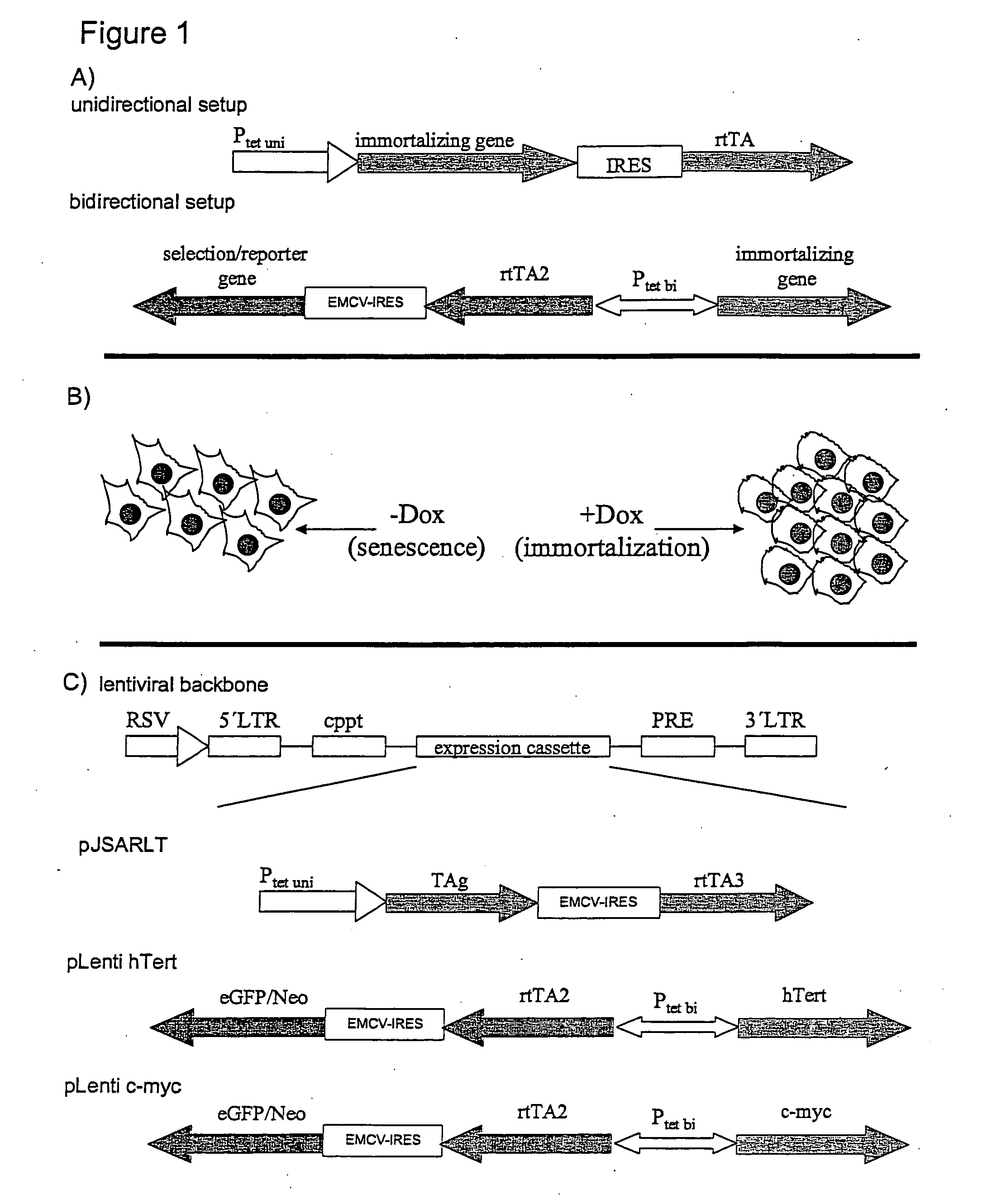
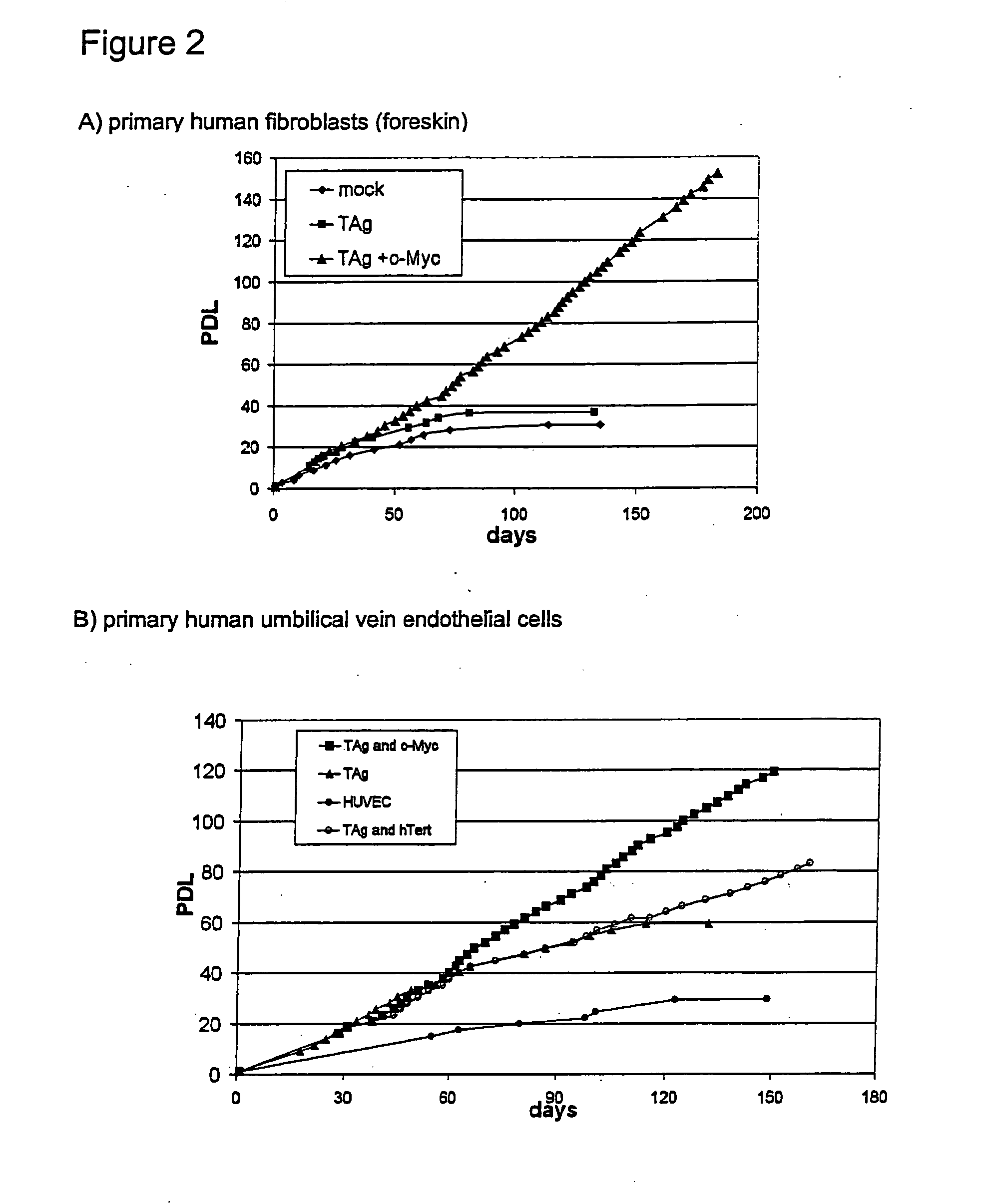
The present invention relates to a method for the generation of immortalized transduced cell lines susceptible to senescence whereby the senescence is exogenously inducible and said cells can switch severalfold between the senescent stage and the immortalized stage. In particular, the present invention relates to a method for the generation of immortalized transduced cell lines susceptible to senescence wherein two or more different immortalizing gene sequences have been incorporated whereby said immortalizing gene sequences are regulated by regulators controlled via exogenous means. In a further aspect, the present invention relates to immortalized transduced cell lines obtainable with said method. In addition, methods for screening molecules influencing senescence of cells are provided as well as kits for conducting the same.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF
Immortalized telocytes system and construction method thereof
ActiveCN107034238AResolve separabilityAddressing Purity IssuesGenetically modified cellsVirus peptidesAntigenCulture mediums
The invention relates to a construction method of an immortalized telocytes system. The construction method comprises the following steps: (1) digesting shorn off mouse lung tissue block in type-II collagenase, carrying out filtering and centrifuging, collecting cyte precipitate, culturing the cyte precipitate in a culture medium, after fibrocytes are attached to a wall, transferring supernate to another culture dish, culturing for 12 hours, then changing liquid, and continuing culturing for 3-5 days; (2) taking a trace spearhead as a cyte scraper to scrape off the fibrocytes, and after scraping off the fibrocytes repeatedly, observing a specificity structure telopode and identifying telocytes by an immune marker; and (3) taking the telocytes as host cells, infecting the host cells by using a retroviral vector which contains SV-40-large tumor antigen, and carrying out passage, screening and identifying. Even if the immortalized telocytes system is cultured to the 50th generation, the state is still stable, and therefore, the problem that the purity and the stability of the telocytes cannot be guaranteed by repeated separation and extraction in the prior art.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Preparation method of immortalized human cartilage endplate stem cell line and use of immortalized human cartilage endplate stem cell line
InactiveCN103865877AStrong osteogenic differentiation abilityLow priceMicroorganism based processesFermentationBone tissue engineeringStem cell line
The invention belongs to the technical field of cell engineering, and particularly relates to a preparation method of an immortalized human cartilage endplate stem cell line and use of the immortalized human cartilage endplate stem cell line. The invention aims to solve the technical problem of providing an effective preparation method for constructing the immortalized human cartilage endplate stem cell line. The technical scheme is as follows: the preparation method for constructing the immortalized human cartilage endplate stem cell line by adopting the lentiviral transfection technology comprises the following steps: a, constructing a SV40T antigen virus vector; b, carrying out gene transfection of a human endplate stem cell on SV40T antigen; and c, passaging and sieving to obtain the immortalized human cartilage endplate stem cell line. The invention further provides the use of the immortalized human cartilage endplate stem cell line prepared by the method in preparation of bone tissue engineering materials. The immortalized human cartilage endplate stem cell line provided by the invention can be applied to preparation of a tissue-engineered bone and clinically provides a seed cell of the bone tissue engineering low in price and strong in osteogenic capability for the patients with a series of long bone defects and bone nonunion.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
Recoverable immortalized rat bone marrow mesenchyme stem cell as well as preparation method and application thereof
InactiveCN102250841AHave biological propertiesEnhance in vitro proliferative abilityFermentationGenetic engineeringHypoxic ischemic brain injuryBrain damage
The invention discloses a recoverable immortalized rat bone marrow mesenchyme stem cell. The cell carries an SV40T (Simian Virus 40T) gene and a hygromycin resistance gene and two ends of the SV40T gene also have orthokinetic Loxp sites. The preparation method of the recoverable immortalized rat bone marrow mesenchyme stem cell comprises the following steps of: co-transfecting an HEK293 (Human Embryo Kidney 293) cell by SSR69 (Simple Sequence Repeat 69) plasmids and pAmhpo plasmids and screening by the hygromycin to obtain the recoverable immortalized rat bone marrow mesenchyme stem cell with hygromycin resistance; the cell has the same biological property with a primary rat and also has the advantages of strong external multiplication capacity, stable biological property, capability of being recovered to a previous state, good biological safety and the like; and the cell provided by the invention can be used as a seed cell induced and differentiated by nerves and a seed cell for treating hypoxicischemic brain damage of a newly-born rat.
Owner:CHILDRENS HOSPITAL OF CHONGQING MEDICAL UNIV
Immortalized yak rumen epithelial cell line and construction method thereof
ActiveCN114058592AMethods for Enriching Isolation and CultureProliferation in good shapeGastrointestinal cellsVirus peptidesNutritionSignalling pathways
The invention discloses an immortalized yak rumen epithelial cell line and a construction method thereof. The preservation number of the cell line is CCTCC No. C2021245, and the cell line is classified and named as the immortalized yak rumen epithelial cell line SV40T-YREC-hTERT. The method comprises the following steps: performing adherent culture on tissue digested by primary yak rumen epithelial papilla type I collagenase to obtain primary rumen epithelial cells; with the cells as host cells, infecting the cells with lentiviruses carrying SV40T and hTERT genes; and performing screening and enlarged culture to obtain the immortalized yak rumen epithelial cell line. Through the establishment of the cell line, the defects that the primary rumen epithelial cells are difficult to culture and the number of passage times is limited are overcome, and yak source cell line resources are enriched; and the cell line can be stably passaged, can keep the morphological characteristics and normal functions of the primary rumen epithelial cells, and provides a useful in-vitro cell model for researching rumen epithelial nutrition digestion, absorption and metabolism, cell signal path mechanisms and the like.
Owner:SICHUAN AGRI UNIV
Methods and compositions for modulating telomerase reverse transcriptase (TERT) expression
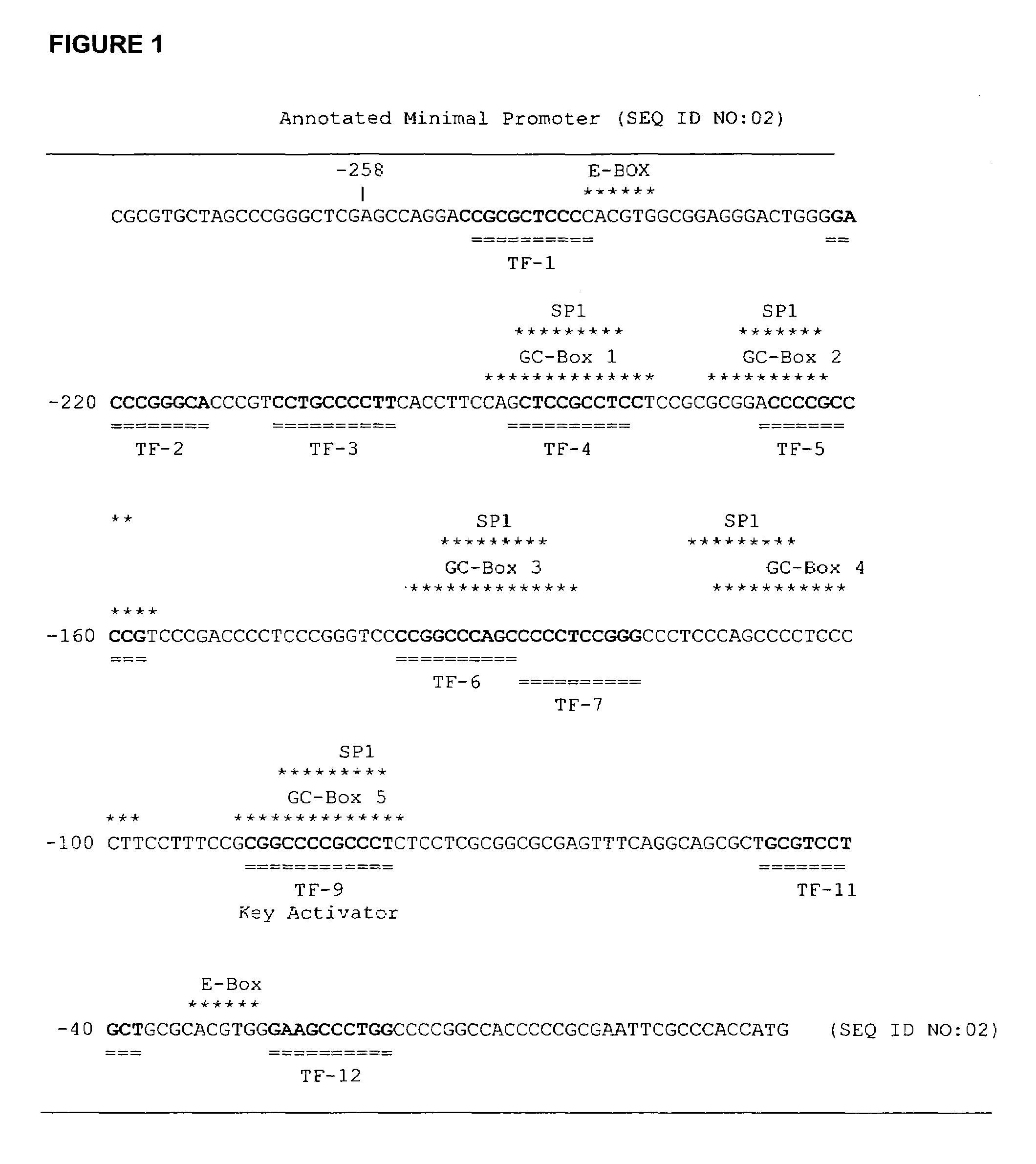
Methods and compositions are provided for modulating, e.g., increasing or decreasing, the expression of telomerase reverse transcriptase (TERT). In the subject methods, the binding interaction of the GC-Box 5 repressor site with a repressor protein (or protein complex including the same) is modulated to achieve the desired change in TERT expression. The subject methods and compositions find use in a variety of different applications, including the immortalization of cells, the production of reagents for use in life science research, therapeutic applications; therapeutic agent screening applications; and the like.
Owner:SIERRA SCIENCES
Periplaneta americana tissue repair factor PA1 and application thereof
ActiveCN111484549AImprove repair effectPromote proliferationCosmetic preparationsPeptide/protein ingredientsTissue repairFibroblast
The invention relates to the field of medicines and daily chemicals, in particular to a periplaneta americana new polypeptide for promoting tissue repair and application of the periplaneta americana new polypeptide, the structure of the periplaneta americana new polypeptide is shown as a formula I, and the periplaneta americana new polypeptide is named as a tissue repair factor PA1. The new polypeptide can promote proliferation of mouse embryo fibroblasts and migration of human immortalized epidermal cells even at a low concentration, has low toxic and side effects and has a good application prospect, so that the polypeptide can be used for preparing medicines for treating wounds, scalds and ulcers or cosmetics and daily chemical products for improving skin beauty.
Owner:JINAN UNIVERSITY
Method for obtaining bioactive substance composition through immortalized cells
InactiveCN104789590APromotes collagen synthesisPromote proliferationCosmetic preparationsToilet preparationsWrinkle skinOrganism
The invention relates to a method for preparing a composition containing human bioactive substances. The bioactive substances are from immortalized cells. The method concretely comprises the following steps: acquiring the immortalized cells made by using autologous cells, and obtaining the bioactive substance composition in a proper medium under proper culture conditions. The bioactive substance composition can be used in customized cosmetics as a raw material or additive in order to realize beauty treatment and skin maintenance. The bioactive substance composition in a cosmetic composition is from human body cells, has consistent three-dimensional structure and bioactivity with bioactive substances in the human body, has certain safety and good skin compatibility, can effectively obtain cell growth factors and other bioactive substances, and can improve cell collagen synthesis, promote fibroblast proliferation and inhibit UV light induced keratinocyte proliferation. The cosmetic composition is expected to be used for resisting wrinkles, beautifying skins, healing wounds and removing scars.
Owner:张烨
Fibroblasts derived from multiple tissue sources of native dog through primary isolated culture and immortalization construction method of fibroblasts
PendingCN112574946AHigh activitySimple and fast operationCell dissociation methodsArtificial cell constructsDiseaseMuscle tissue
The invention provides fibroblasts derived from multiple tissue sources of a native dog through primary isolated culture and an immortalization construction method of the fibroblasts, and belongs to the technical field of cell culture. Heart tissue, lung tip muscle tissue or leg muscle tissue of the native dog are used as materials and subjected to enzymolysis and separation of pancreatin and / or collagenase II, and myocardial fibroblasts, lung fibroblasts and myofibroblasts with typical fibroblast morphological characteristics are obtained. The myocardial fibroblasts, the pulmonary fibroblastsand the myofibroblasts which are subjected to primary isolated culture are transfected by SV40T virus liquid, and then puromycin screening culture and passage are performed to obtain immortalized myocardial fibroblasts, pulmonary fibroblasts and myofibroblasts. Compared with primary culture cells, the immortalized constructed cells are higher in growth speed and remarkably improved in activity, and a material basis is provided for dogs in the aspects of scientific research, disease research and the like.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Telomerase immortalized human fetal forebrain neural precursor cell line and construction method thereof
InactiveCN101353646AHave electrophysiological propertiesIncrease telomerase activityNervous disorderMammal material medical ingredientsCholinergic cellsTelomerase
The invention relates to a cell line of precursor of forebrain nerve of a telomerase immortalization human embryo and an establishing method and application thereof; the cell line is preserved in China culture collection and management committee general biology center in 21st February 2008, with the collection number of CGMCC No.2372. The cell line of the invention is named as hSN12W-TERT; the cell line expresses growth factor receptor of human nerves and green fluorescent protein, has high activity of telomerase, double core type than normal human, continuous passage culture in vitro of 36 months with 45th generation at the present and population doubling of 100 to 200 times and has the property of contact inhibition and density inhibition; the neuron which is formed by differentiation of the cell line has the property of electrophysiology; the cell line can be differentiated into Gamma-aminobutyric acid (GABA) cholinergic neuron under the induction of bone marphogenic protein 2(BMP2).
Owner:首都医科大学北京神经科学研究所
Gene, vector and method for preparing immortalized dendritic cells and immortalized dendritic cells
The invention provides a gene, a vector and a method for preparing immortalized dendritic cells and the immortalized dendritic cells, belonging to the technical field of genetic engineering. The genefor preparing the immortalized dendritic cells is obtained by connecting an ST40 gene with a TAX2 gene through a linker, wherein the nucleotide sequence of the ST40 gene is as shown in SEQ ID No. 1; and the nucleotide sequence of the TAX2 gene is shown as SEQ ID No. 2. The immortalized dendritic cells prepared from the genes provided by the invention comprise HLA-A0201, and covers nearly nine types of common HLA-A1101, HLA-A2402, HLA-A0301 and the like of Chinese population, so the application range is greatly enlarged.
Owner:BEIJING DCTY BIOTECH CO LTD
Immortalized rat marrow stroma cell system and preparation method thereof
InactiveCN101255407AImprove proliferative abilityRealization of immortalityNervous disorderUnknown materialsBone Marrow Stromal CellKaryotype
The invention provides a immortal stromal cell systme of rat's marrow and preparation thereof. Immortal stromal cell of rat's marrow can be obtained in short time by the inventive method. The inventive hTERT immortal stromal cell systme of rat's marrow is stable, can be sequentially passed through generations and keep diploid karyotype and characteristics of normal stromal cell of rat's marrow, has extremely strong proliferation ability, and realize immortalization. Immortal stromal cell system of rat's marrow established by the inventive method can conveniently and quickly obtain large amount of stromal cell of rat's marrow with diploid karyotype. This no doubt has great promotion to research of introduction and bone marrow stromal cell in vitro and in vivo.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Preparation method and applications of immortalized duck embryo hepatic cell line
ActiveCN104726409ASimple cultivation conditionsEasy to controlMicroorganism based processesAntiviralsDigestionDisease
The invention provides a preparation method of an immortalized duck embryo hepatic cell line. The preparation method comprises following steps: (1) duck embryo liver is subjected to digestion with trypsase, and an obtained digestive juice is subjected to culturing so as to obtain primary cultured duck embryo hepatic cells; (2) lipidosome transfection is adopted, and eukaryotic expression plasmids used for coding hTERT and Marker genes are introduced into the primary cultured duck embryo hepatic cells; (3) obtained transfected duck embryo hepatic cells are subjected to culturing, and hTERT positive clones are selected with G418; and (4) selected hTERT positive clones are subjected to culturing, and the immortalized duck embryo hepatic cell line is obtained after more than 50 generations of continuous culturing. The immortalized duck embryo hepatic cell line is capable of maintaining relatively excellent activity after more than 50 generations of in vitro continuous cell culture; division and proliferation can be maintained; no aging or apoptosis is caused; and immortalization of the immortalized duck embryo hepatic cell line is realized. The immortalized duck embryo hepatic cell line is regular in cell morphology; culturing conditions are simple and are easy to control; using is convenient; and an excellent carrier is provided for research on duck disease, especially on duck hepatitis virus.
Owner:PU LIKE BIO ENG
Human bronchial epithelial cell strain HBE-TT
ActiveCN105483087AMaintain normal featuresNo features of malignant transformationForeign genetic material cellsMycoplasma contaminationPulmonary disease
The invention a novel human bronchial epithelial cell strain HBE-TT, collected in China Center for Type Culture Collection (Wuhan) under CCTCC NO: C201560. The cell strain HBE-TT is verified to be good in purity, free of sundry cell contamination and differed from all existing other cell strains. In addition, the immortalized cells retain typical features of primary bronchial epithelial cells and are free of mycoplasma contamination and vicious transformation features, and it is suggested that cells HBE-TT can retain trait stability during in-vitro subculture. The cells HBE-TT are expected to be a forcible tool for the deep discussion on occurrence and development molecular mechanism of lung diseases caused by environmental toxic factor exposure and for pharmacological study, and the cells are significant to the study on health damage effect of toxic factors on human lung as well as risk estimation and new drug development.
Owner:SUN YAT SEN UNIV
Method for large-scale culture of immortalized porcine hepatocyte
The invention discloses a large-scale culture method of immortalized pork liver cells, which comprises: the immortalized pork liver cells collected are inoculated in a spinner bottle, rotating culture is carried out in a three-layer culture system, sodium alginate solution with a concentration of 1.5 percent to 2.0 percent is used for adjusting the concentration of the immortalized pork liver cells to 1 to 2.5 mulpied by 10<6> / mL; the immortalized pork liver cells obtained through readjustment of the concentration is prepared into immortalized pork liver cell micro-capsule by one-step method with alginic acid-chitosan; and the invention further discloses a microencapsulized immortalized pork liver cell. The microencapsulized immortalized pork liver cell can be used as liver cell source for the transplantation of biotype artificial liver or liver cell. The HCMV gBn1 antibody prepared has the advantages of strong specificity and high sensitivity.
Owner:ZHEJIANG UNIV
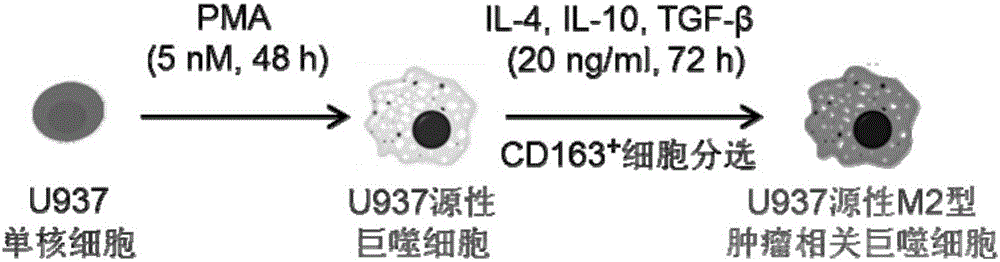
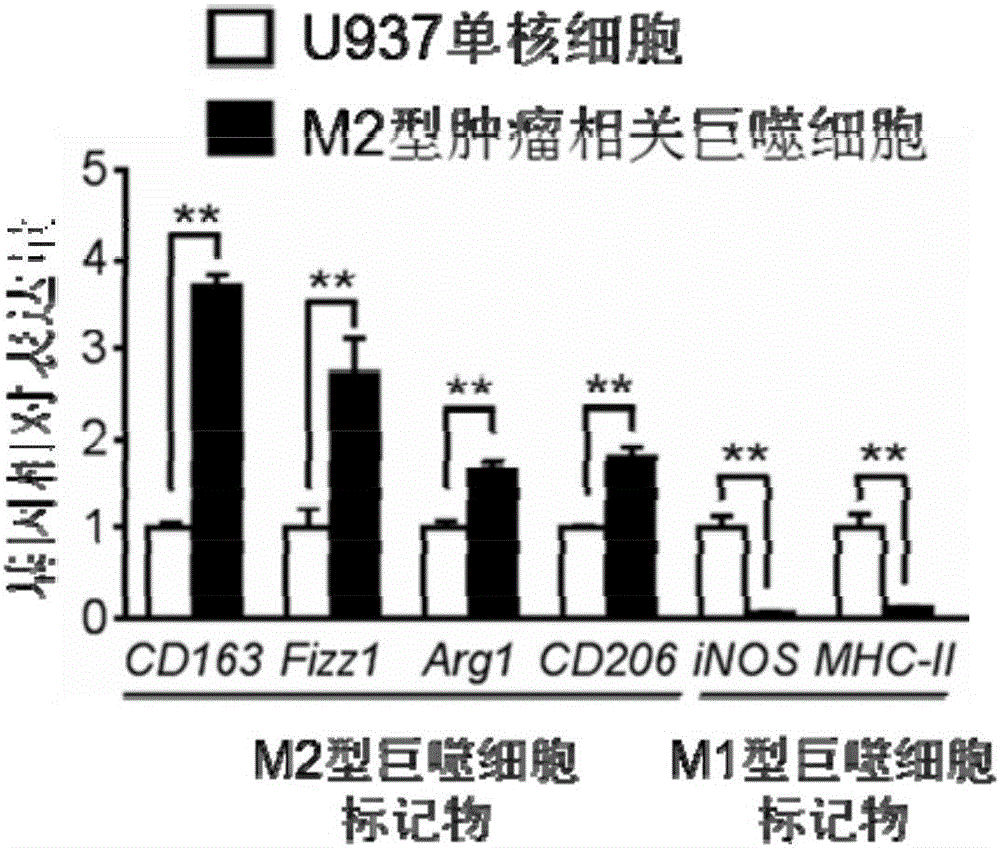
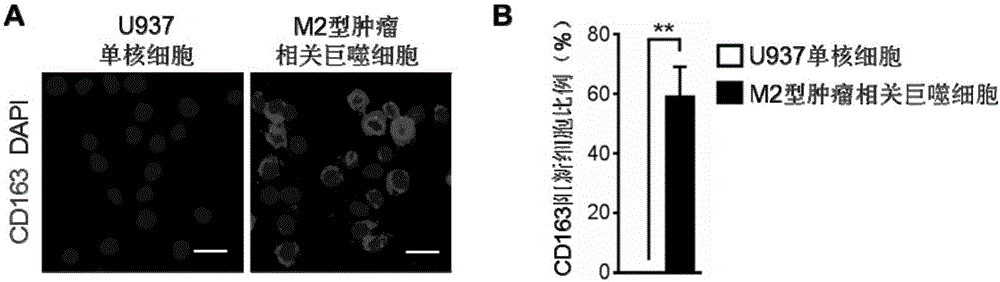
Construction method and application of M2-type tumor-associated macrophage model
InactiveCN106244546APromote growthShortened lifetimeBlood/immune system cellsCell culture active agentsIn vivoWilms' tumor
The invention relates to a construction method and an application of a M2-type tumor-associated macrophage model. In the invention, an immortalized mononuclear cell (for example, a U937 monocytic series) is induced into a tumor-associated macrophage in vitro environment under stimulation of PMA and IL-4, IL-10 and TGF[beta] growth factor; and on the basis of the mentioned before, biological functions and target therapy value of the tumor-associated macrophage in tumor progression are researched in a manner of in-vivo and in-vitro co-cultivation method of the tumor-associated macrophage and tumor cells. The invention provides important tools and experimental measures for tumor immunology fundamental research and therapy measures based on the tumor-associated macrophage.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
Use of human prostate cell lines in cancer treatment
InactiveUS20050249756A1Promote growthSufficient characteristicBacterial antigen ingredientsPeptide/protein ingredientsGamma irradiationHuman prostate
The invention here relates to a product comprised of a cell line or lines intended for use as an allogeneic immunotherapy agent for the treatment of cancer in mammals and humans. All of the studies of cell-based cancer vaccines to date have one feature in common, namely the intention to use cells that contain at least some TSAs and / or TAAs that are shared with the antigens present in patients' tumour. In each case, tumour cells are utilised as the starting point on the premise that only rumour cells will contain TSAs or TAAs of relevance, and the tissue origins of the cells are matched to the tumour site in patients. A primary aspect of the invention is the use of immortalised normal, non-malignant cells as the basis of an allogeneic cell cancer vaccine. Normal cells do not possess TSAs or relevant concentrations of TAAs and hence it is surprising that normal cells are effective as anti-cancer vaccines. For prostate cancer, for example, a vaccine may be based on one or a combination of different immortalised normal cell lines derived from the prostate. The cell lines are lethally irradiated utilising gamma irradiation at 50-300 Gy to ensure that they are replication incompetent prior to use in the mammal or human.
Owner:ONYVAX
Methods for Efficient Immortalization Of Normal Human Cells
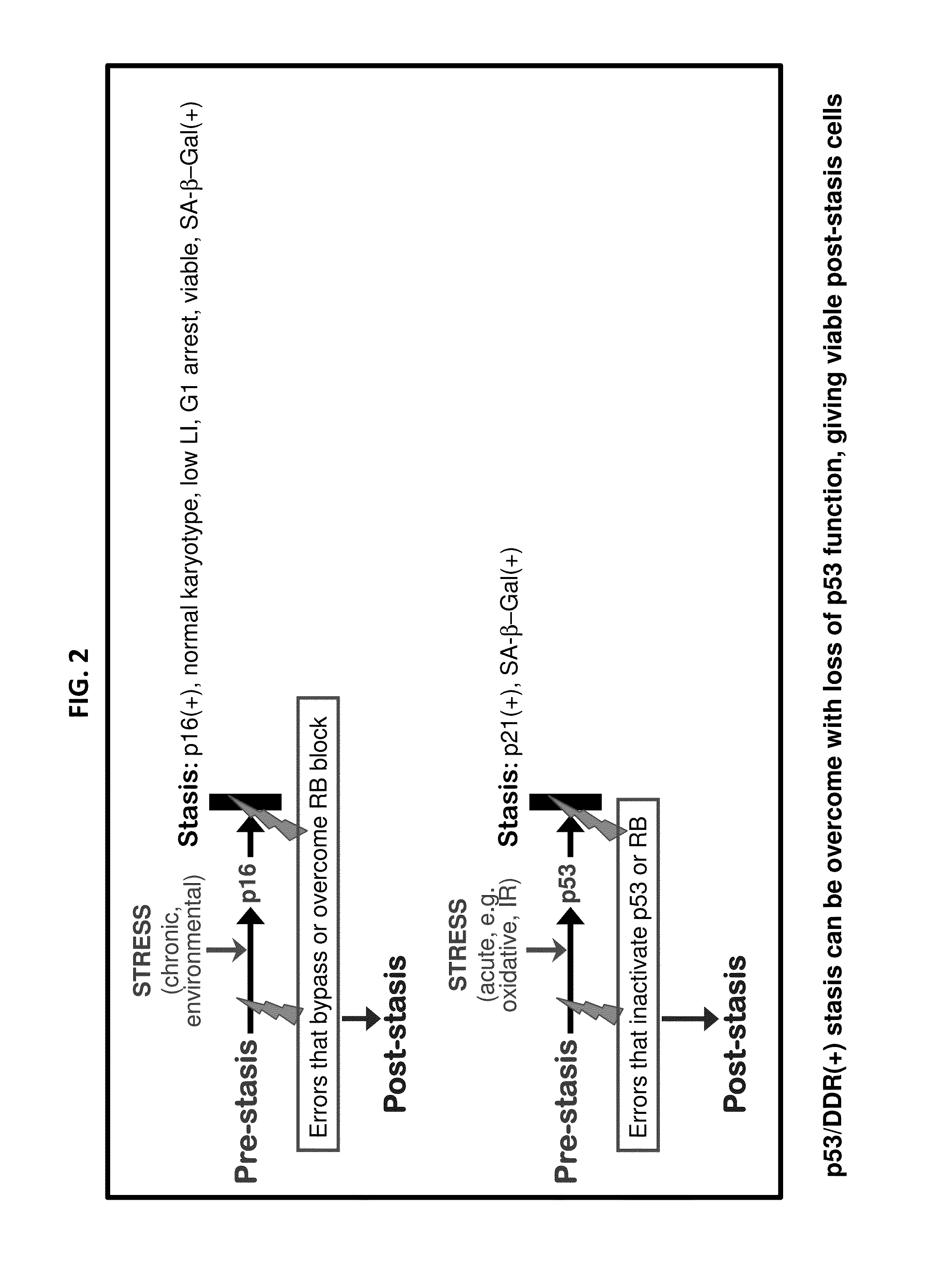
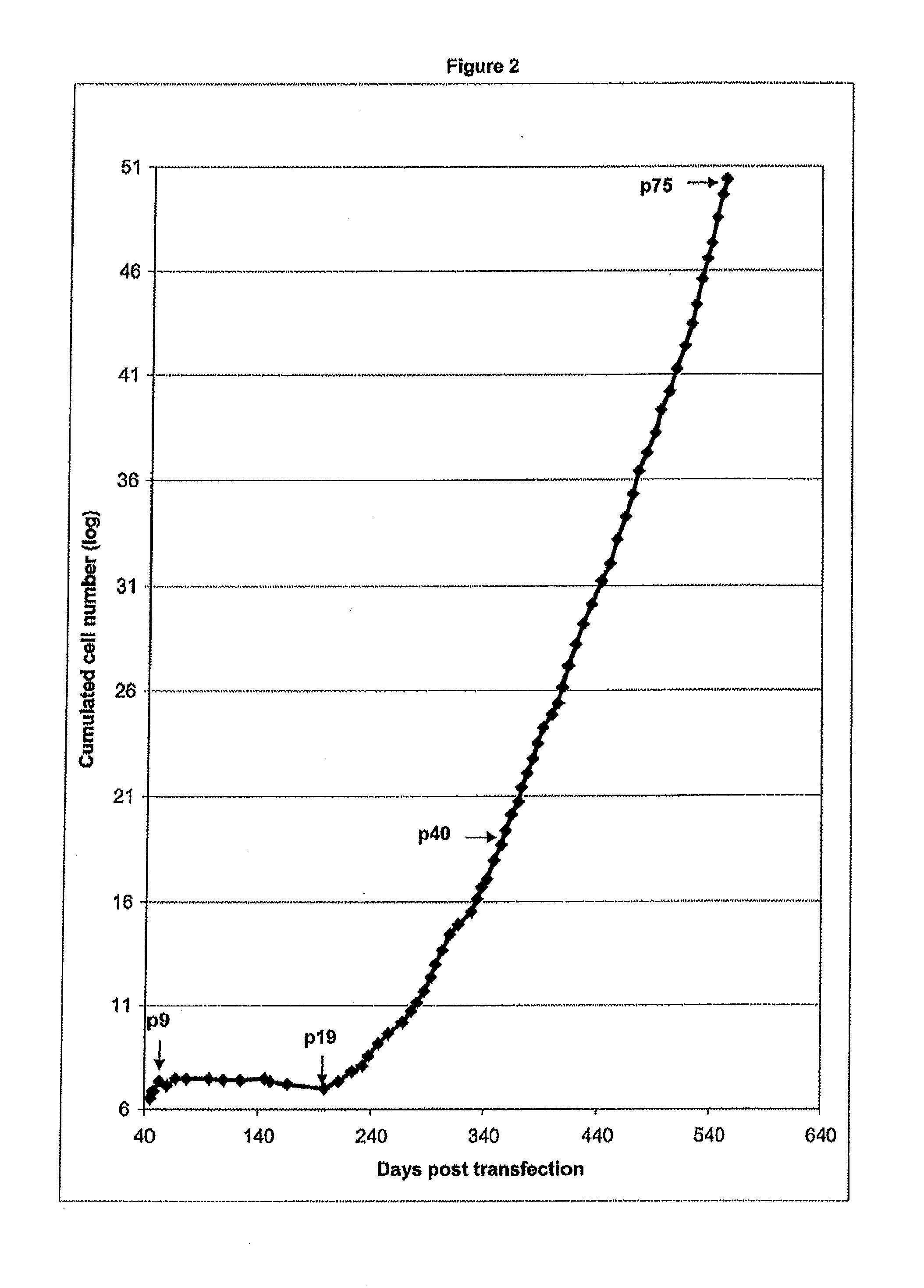
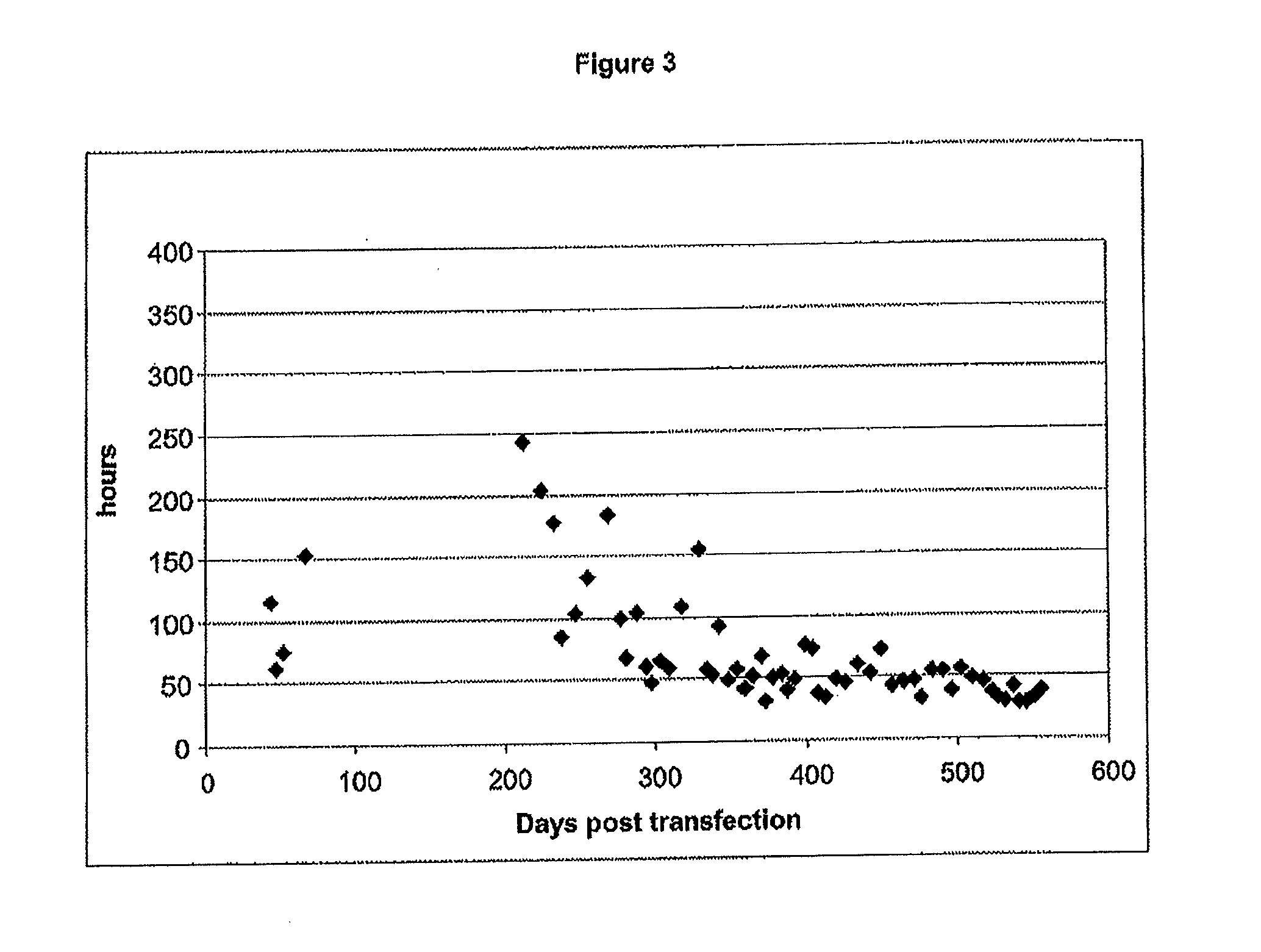
Methods for inducing non-clonal immortalization of normal epithelial cells by directly targeting the two main senescence barriers encountered by cultured epithelial cells. In human mammary epithelial cells (HMEC), the stress-associated stasis barrier was bypassed and the replicative senescence barrier, a consequence of critically shortened telomeres, was bypassed in post-stasis HMEC. Early passage non-clonal immortalized lines exhibited normal karyotypes. Methods of efficient HMEC immortalization, in the absence of “passenger” genomic errors, should facilitate examination of telomerase regulation, immortalization during human carcinoma progression, and methods for screening for toxic and environmental effect on progression.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA +1
Immortalized avian cell lines and use thereof
InactiveUS20110212488A1Easy maintenanceImprove stabilityAntibacterial agentsNervous disorderFlaviviridaeVaccinia
The present invention relates to specific immortalized avian cell lines expressing telomerase reverse transcriptase (TERT), and exhibiting distinct biologics production patterns. More particularly, the present invention relates to immortalized avian cell line capable of either amplifying Flaviviridae but not capable of amplifying Vaccinia virus strain Copenhagen (W—COP) nor Modified Vaccinia virus Ankara (MVA), or capable of amplifying both Flaviviridae and Poxyiridae. The invention further relates to the use of said immortalized avian cell lines and related methods for producing biologics, including viruses and proteins.
Owner:TRANSGENE SA
Method for reprogramming immortalized lymphocyte line into induced pluripotent stem cells
ActiveCN112961833AExpand the value of clinical researchReduce the risk of tumorigenesisGenetically modified cellsNucleic acid vectorGerm layerReprogramming
The invention provides a method for reprogramming an immortalized lymphocyte line into induced pluripotent stem cells. The reprogramming method mainly comprises the step of introducing additional plasmids for expressing exogenous reprogramming factors into the immortalized lymphocytes through a nuclear transformation method, wherein the reprogramming factors comprise OCT3 / 4, shP53, SOX2, KLF4, LIN28 and L-MYC. According to the method, the immortalized lymphocyte line can be efficiently reprogrammed into the iPSC, and the additional plasmids cannot be integrated into the genome of host cells and can be gradually lost along with cell proliferation, so that exogenous expression can be timely silenced, and the cell tumorigenesis risk is reduced. The obtained iPSC has typical stem cell morphology and characteristics similar to ESC, expresses cell stemness genes, and has differentiation potential of differentiating into ectoderm, mesoderm and endoderm tissues. The method is high in reprogramming efficiency and low in tumorigenicity, the clinical research value of the immortalized lymphocyte line is expanded, and the method has application prospects.
Owner:ZHEJIANG UNIV
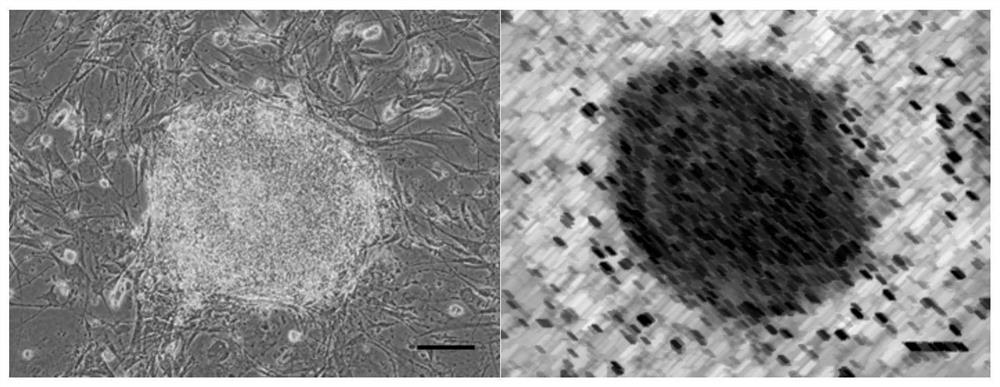
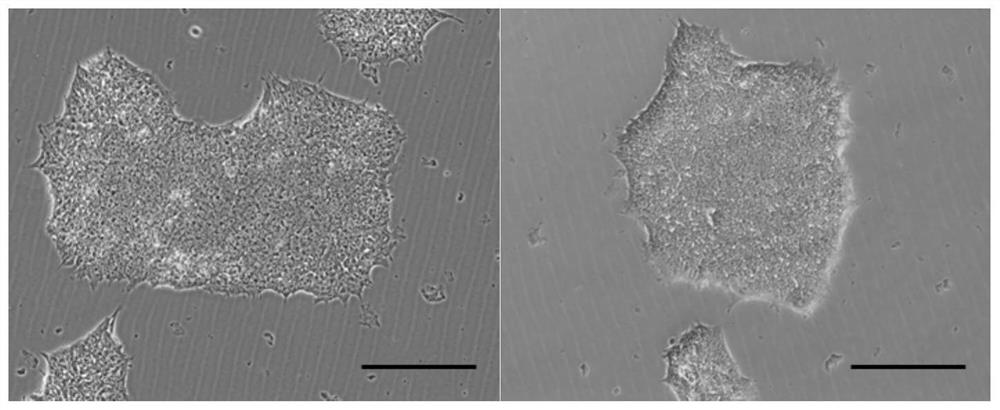
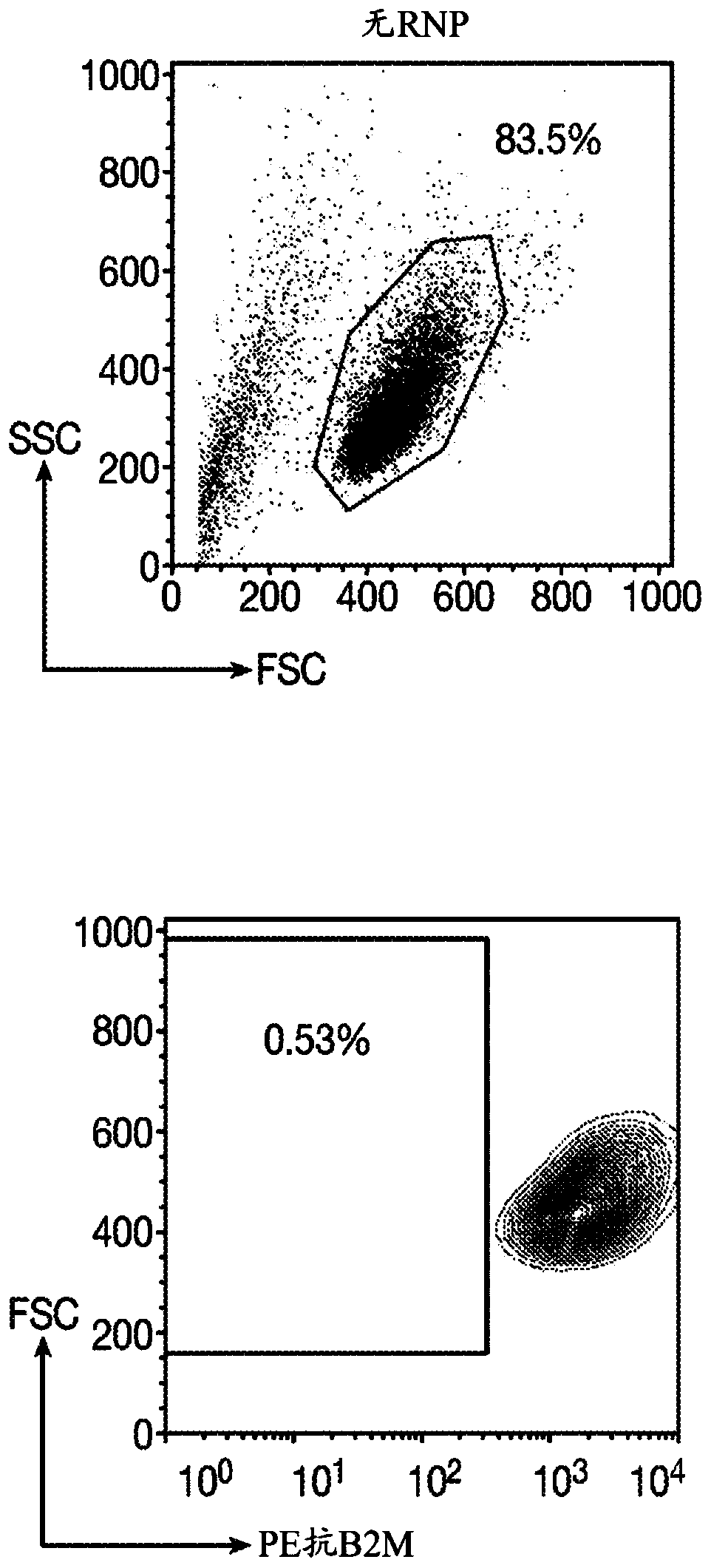
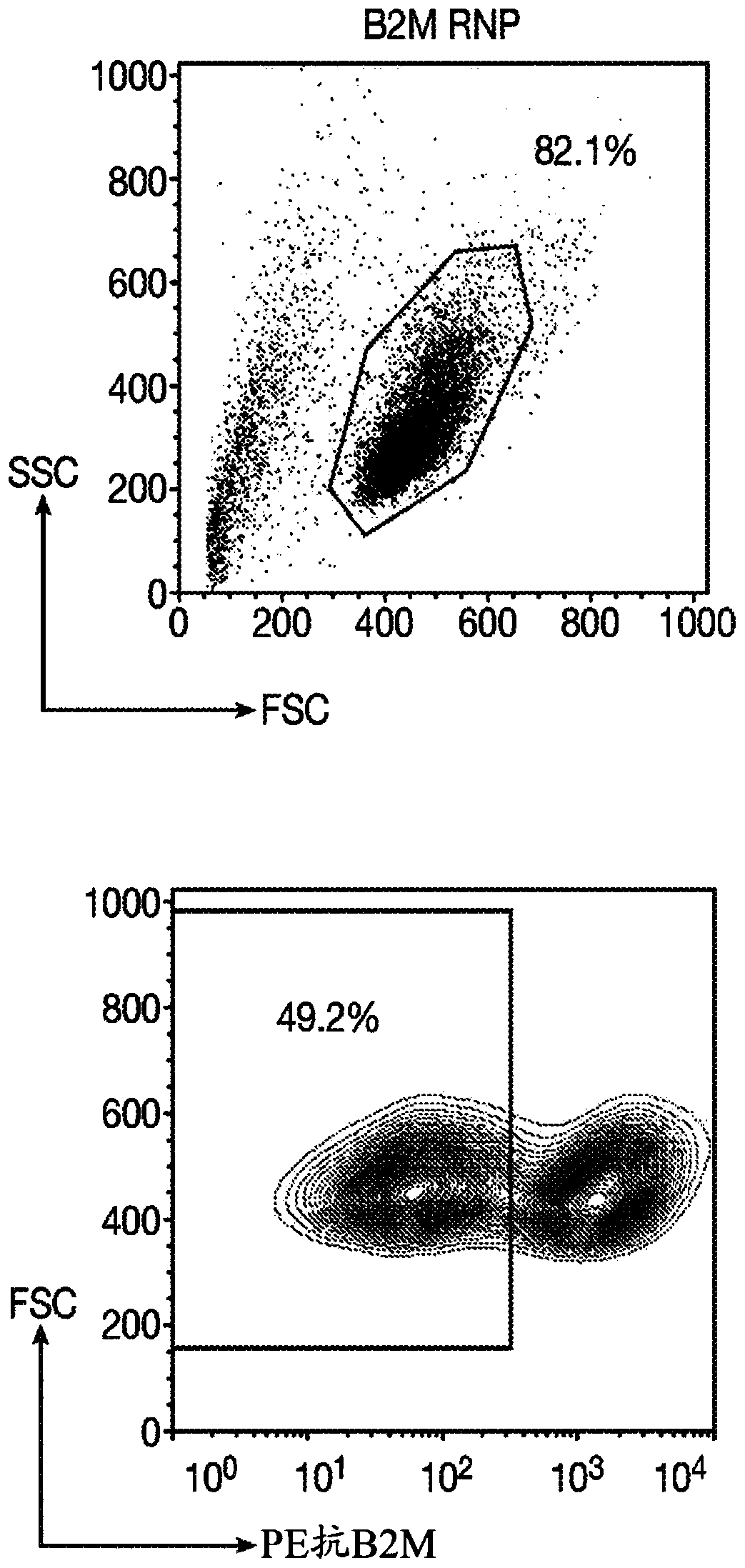
Immortalized car-t cells genetically modified to eliminate t-cell receptor and beta2-microglobulin expression
The present invention pertains to engineered immortalized T-cell lines, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered immortalized T-cell lines of the invention are characterized in that the expression of endogenous T-cell receptors (TCRs) and beta 2-microglobulin (B2M) is inhibited, e.g., by using an endonuclease able to selectively inactivate the TCR and B2M genes in order to render the immortalized T-cells non-alloreactive. In addition, expression of immunosuppressive polypeptide can be performed on those engineered immortalized T-cells in order to prolong the survival of these T-cells in host organisms. Such engineered immortalized T-cells are particularly suitable for allogeneic transplantations, especially because it reducesboth the risk of rejection by the host's immune system and the risk of developing graft versus host disease. The invention opens the way to standard and affordable adoptive immunotherapy strategies using immortalized T-cells for treating cancer, infections and auto-immune diseases.
Owner:JANSSEN BIOTECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com