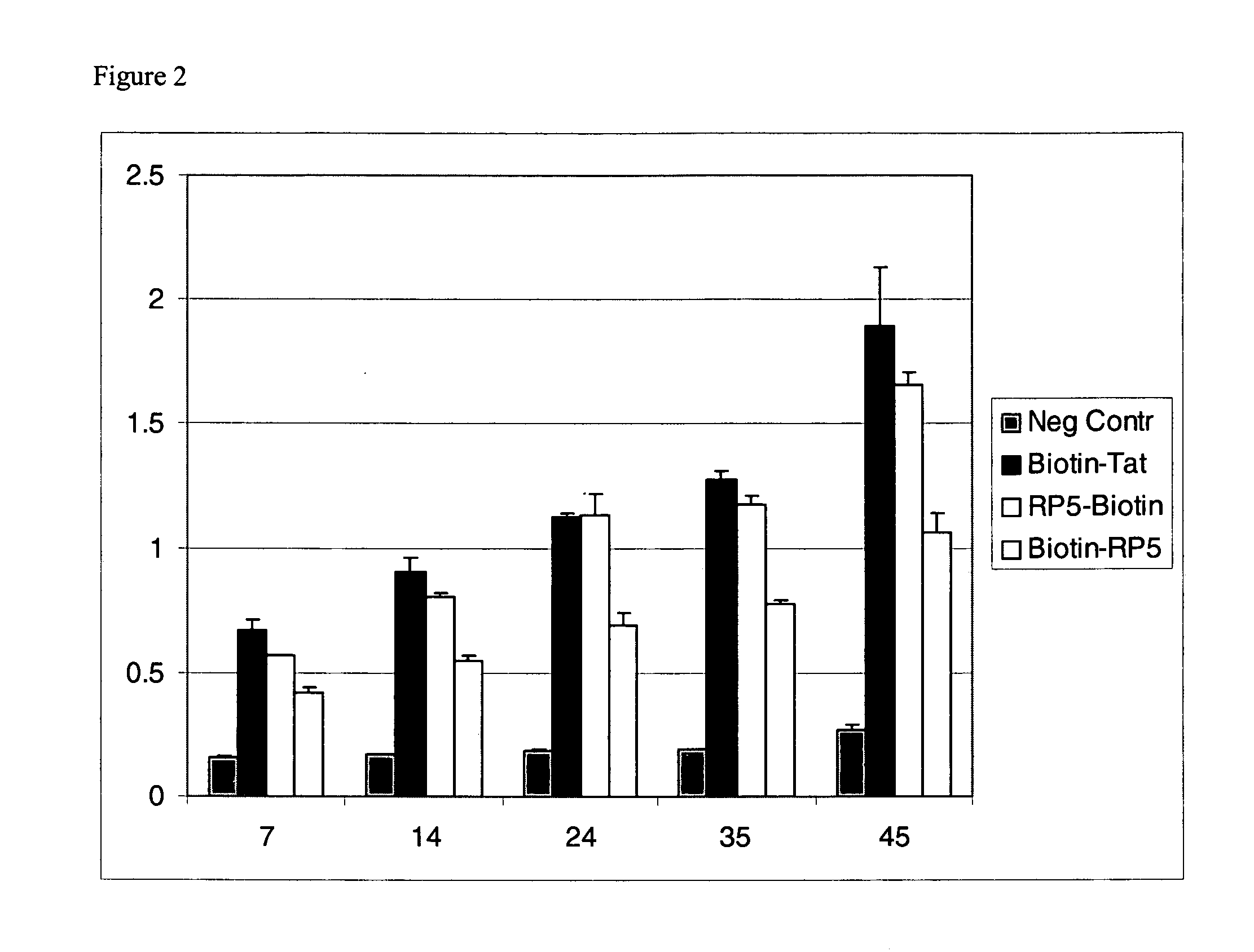
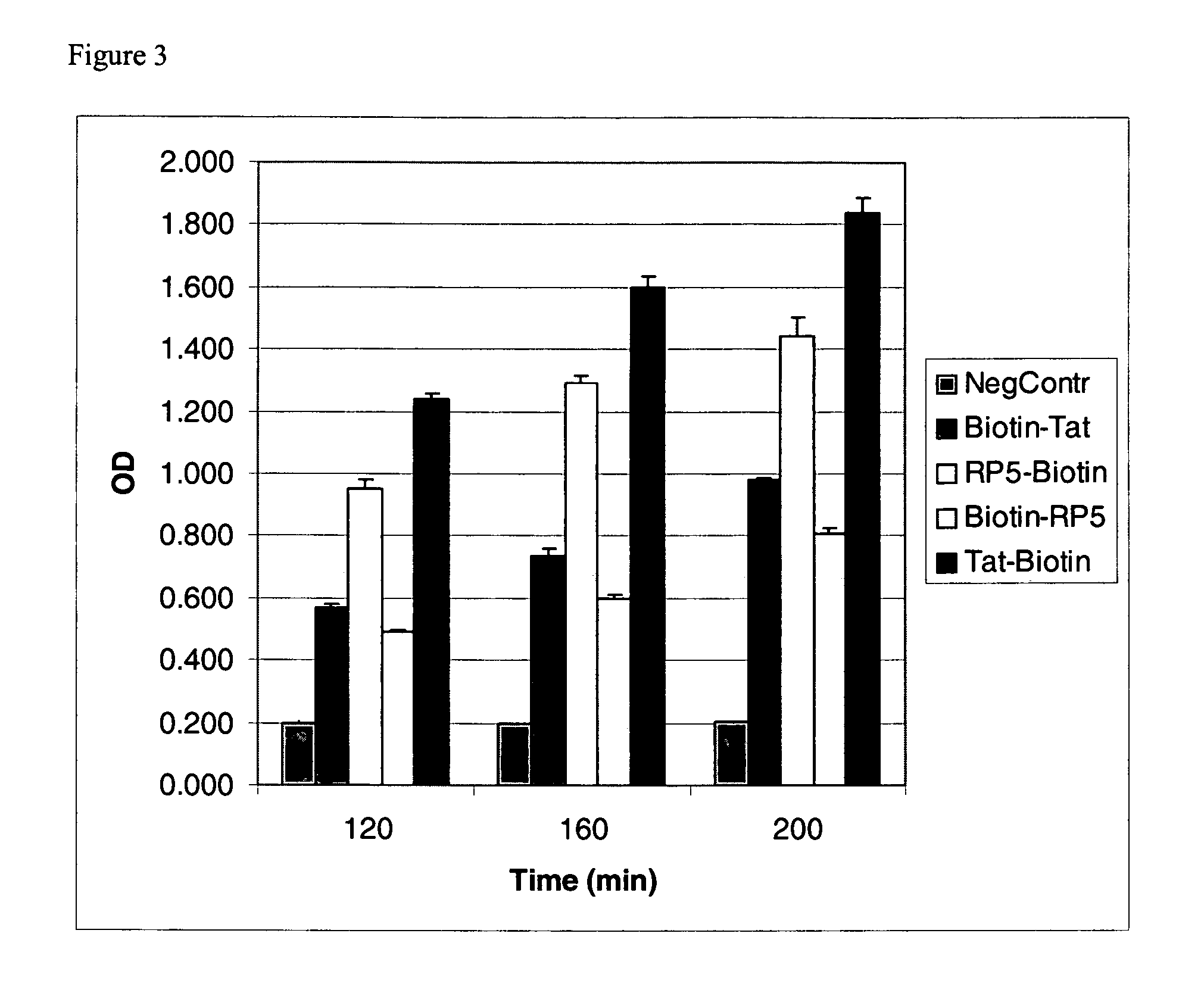
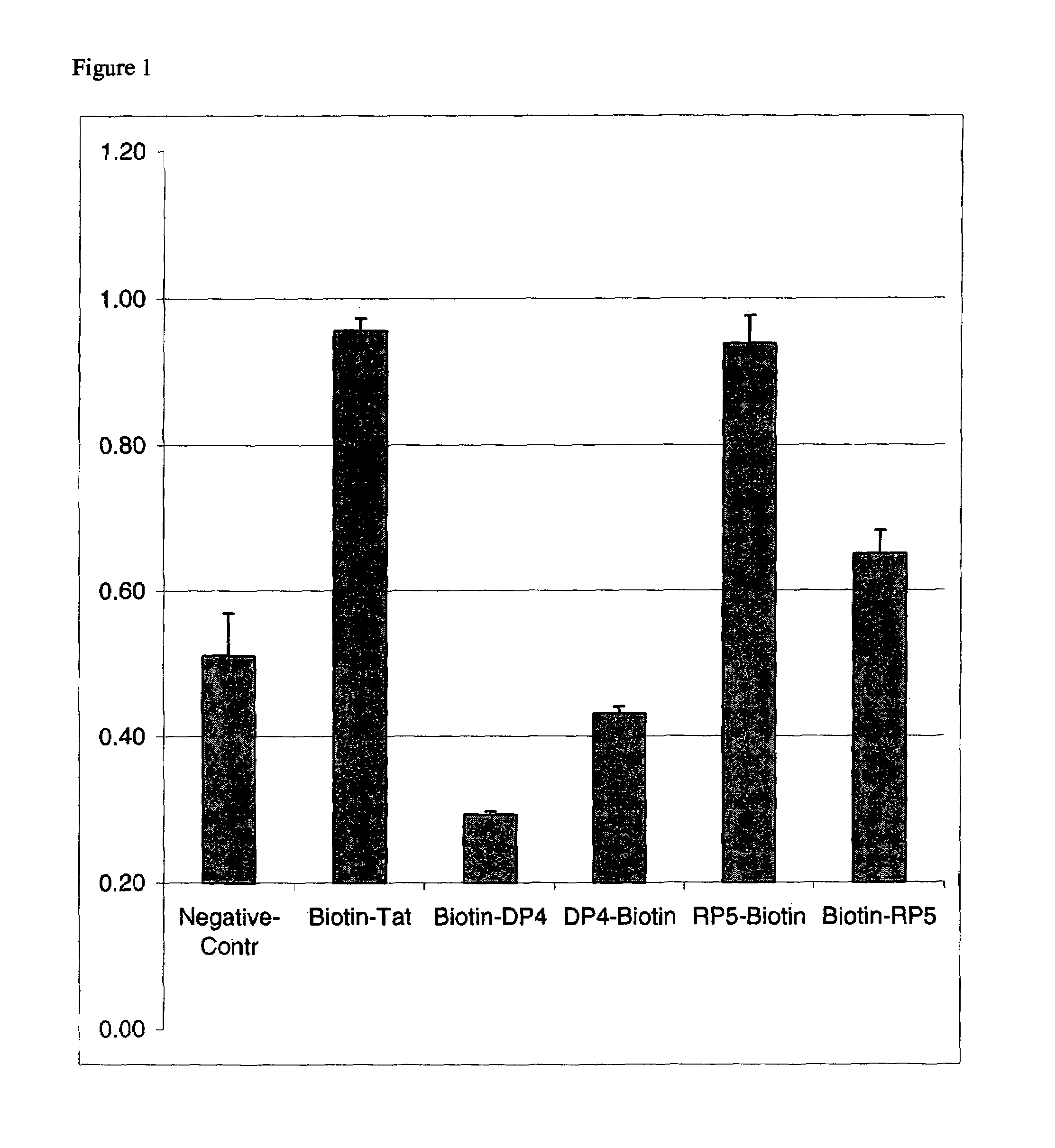
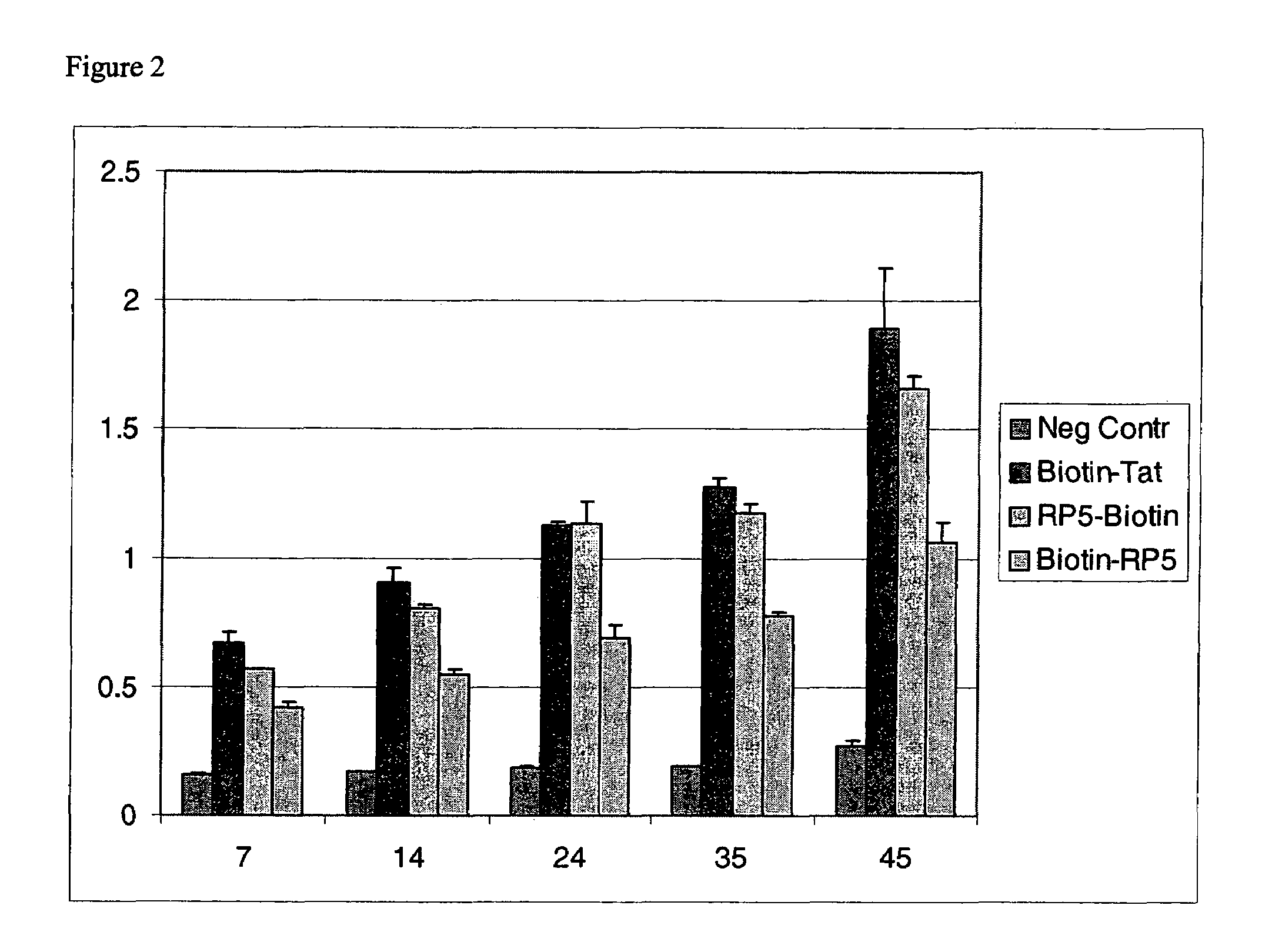
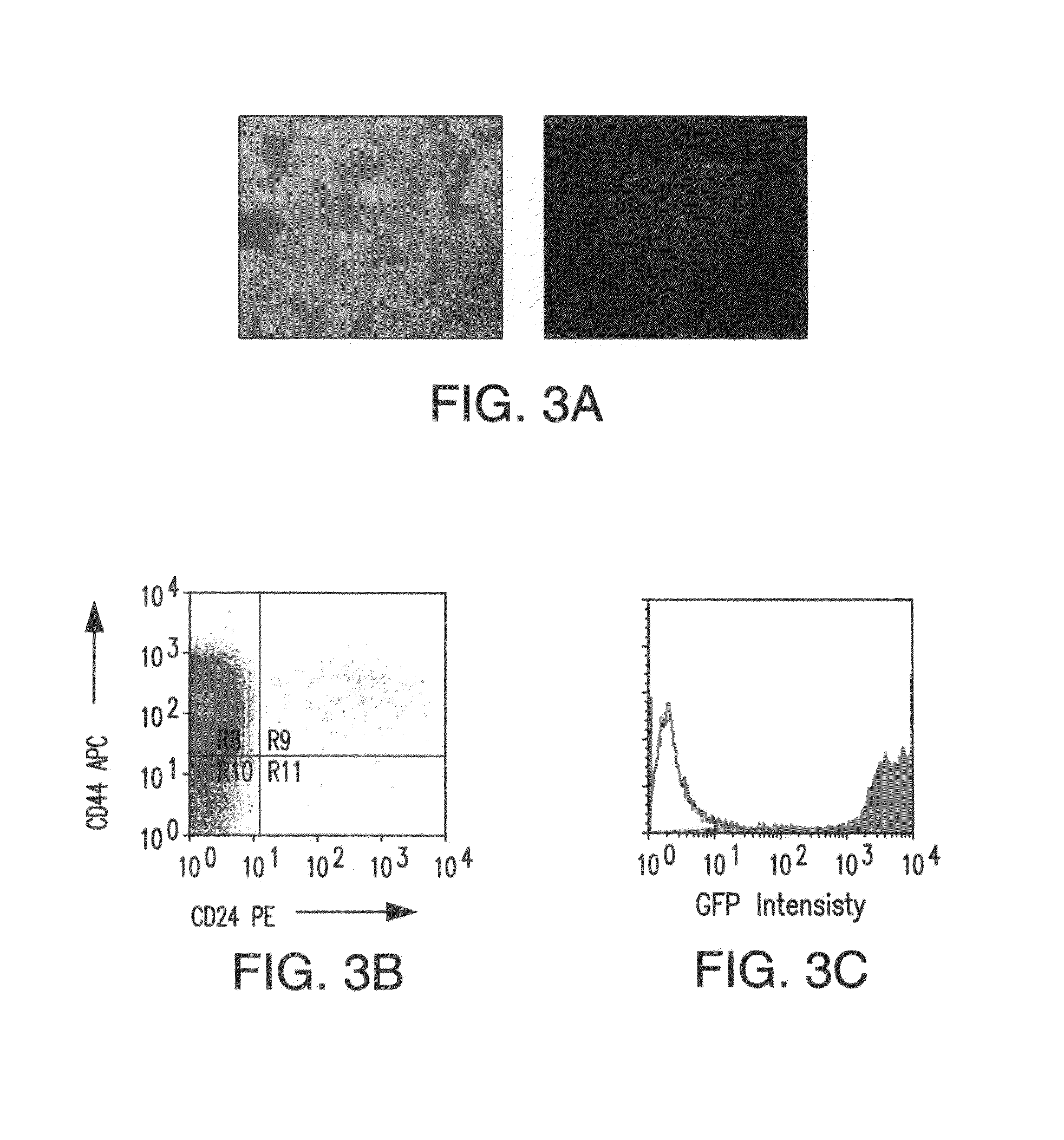
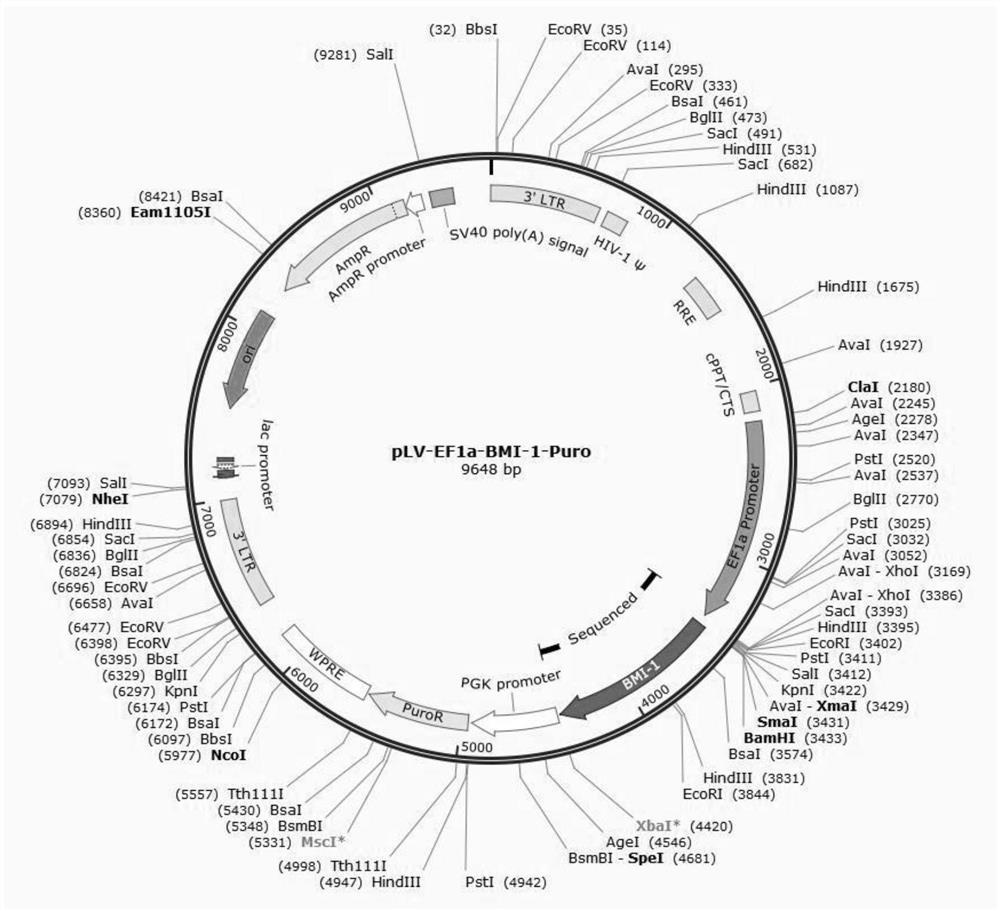
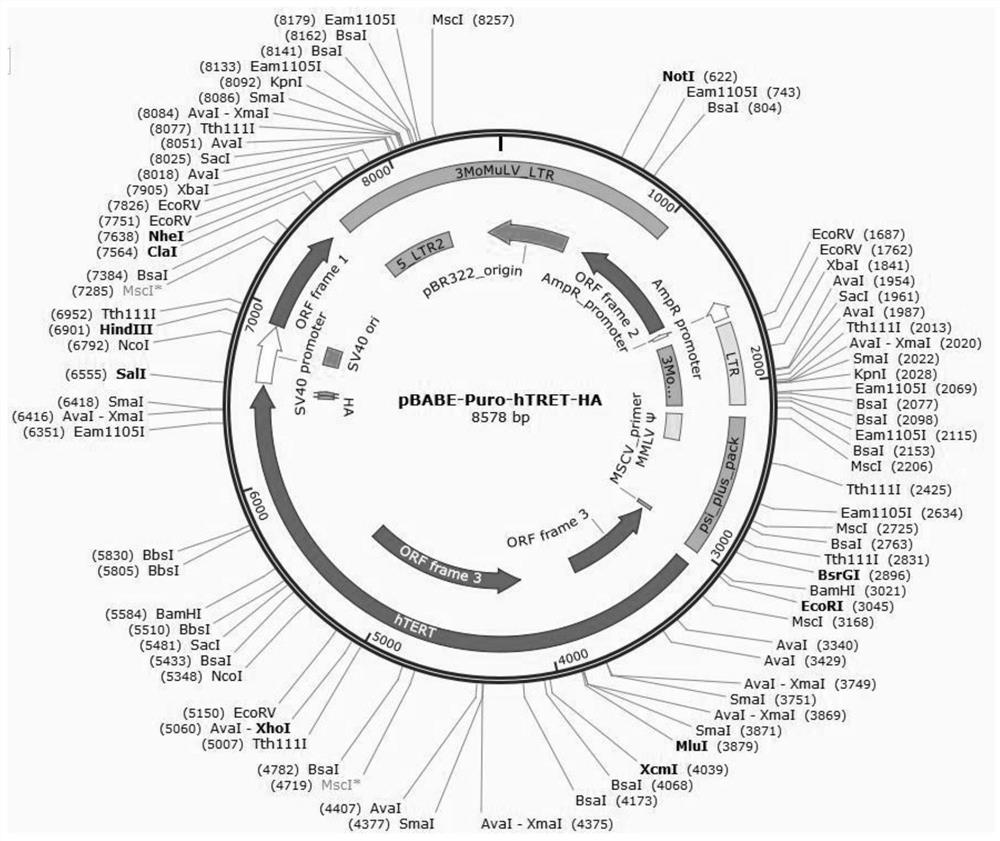
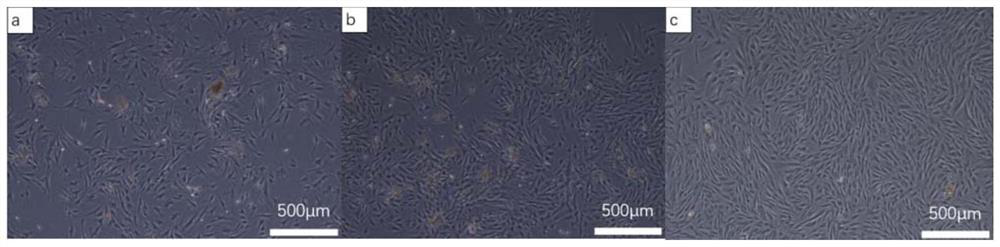
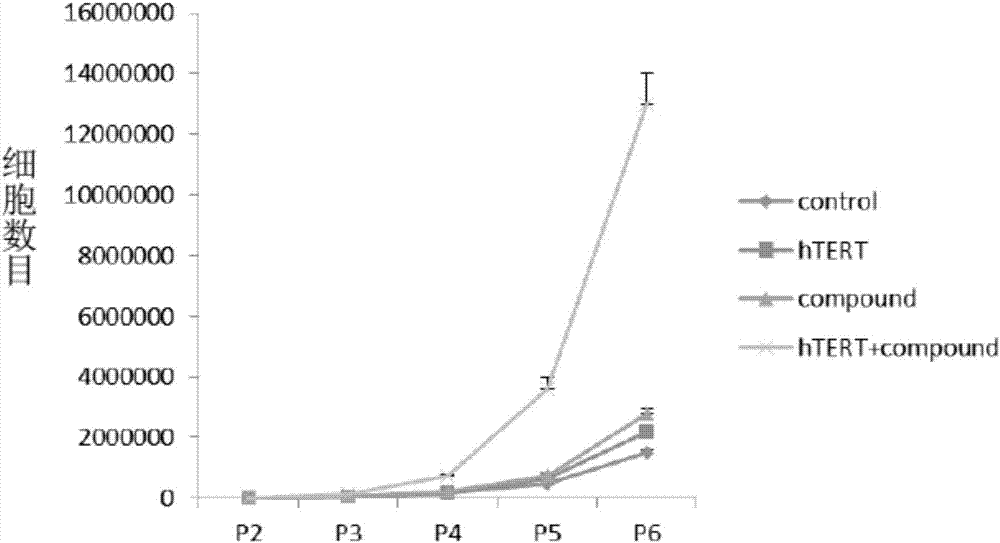
Patents
Literature
34 results about "Cell immortalization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods for immortalizing cells
InactiveUS7399633B2Antibody mimetics/scaffoldsGenetically modified cellsCell Differentiation processCultured cell
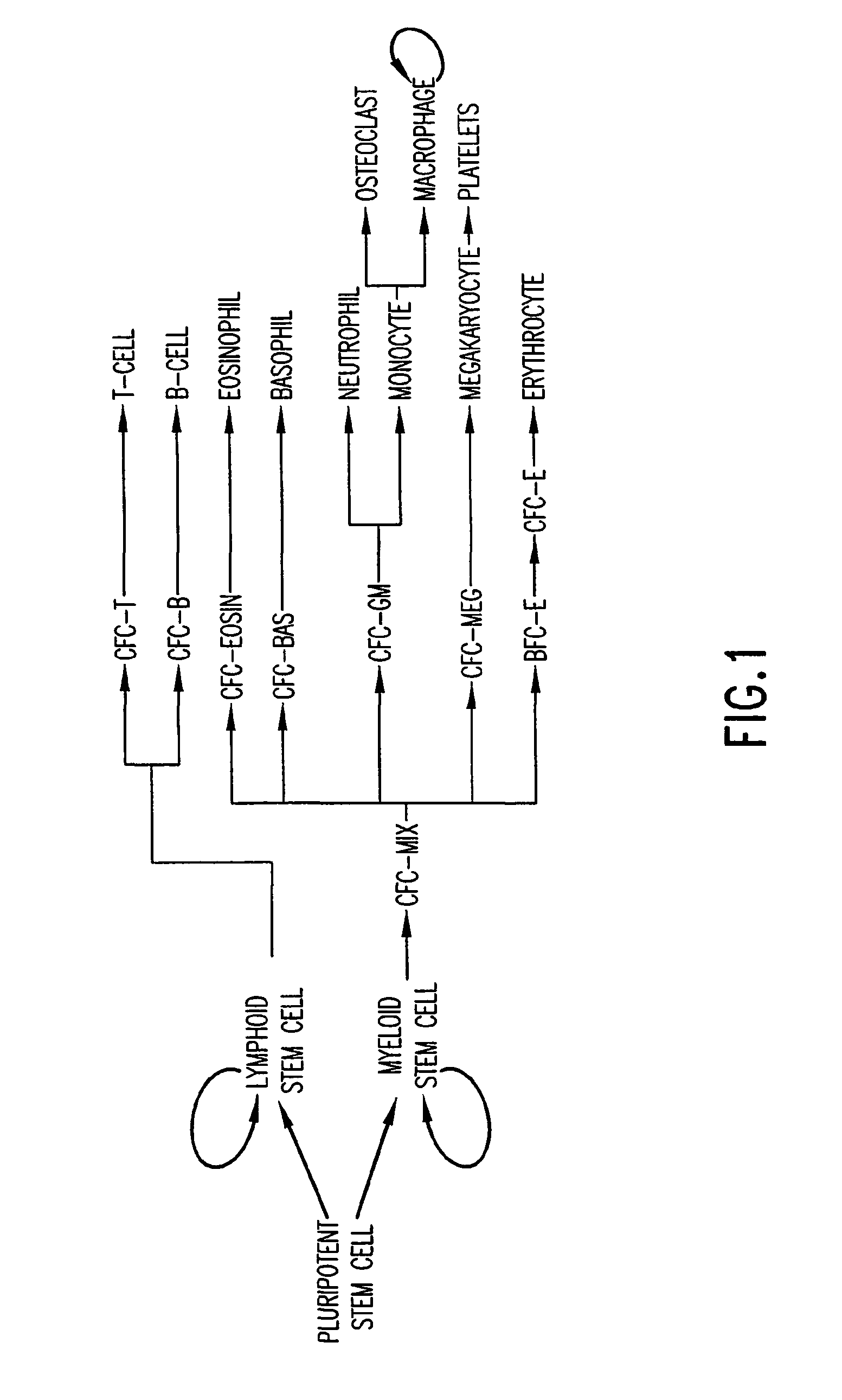
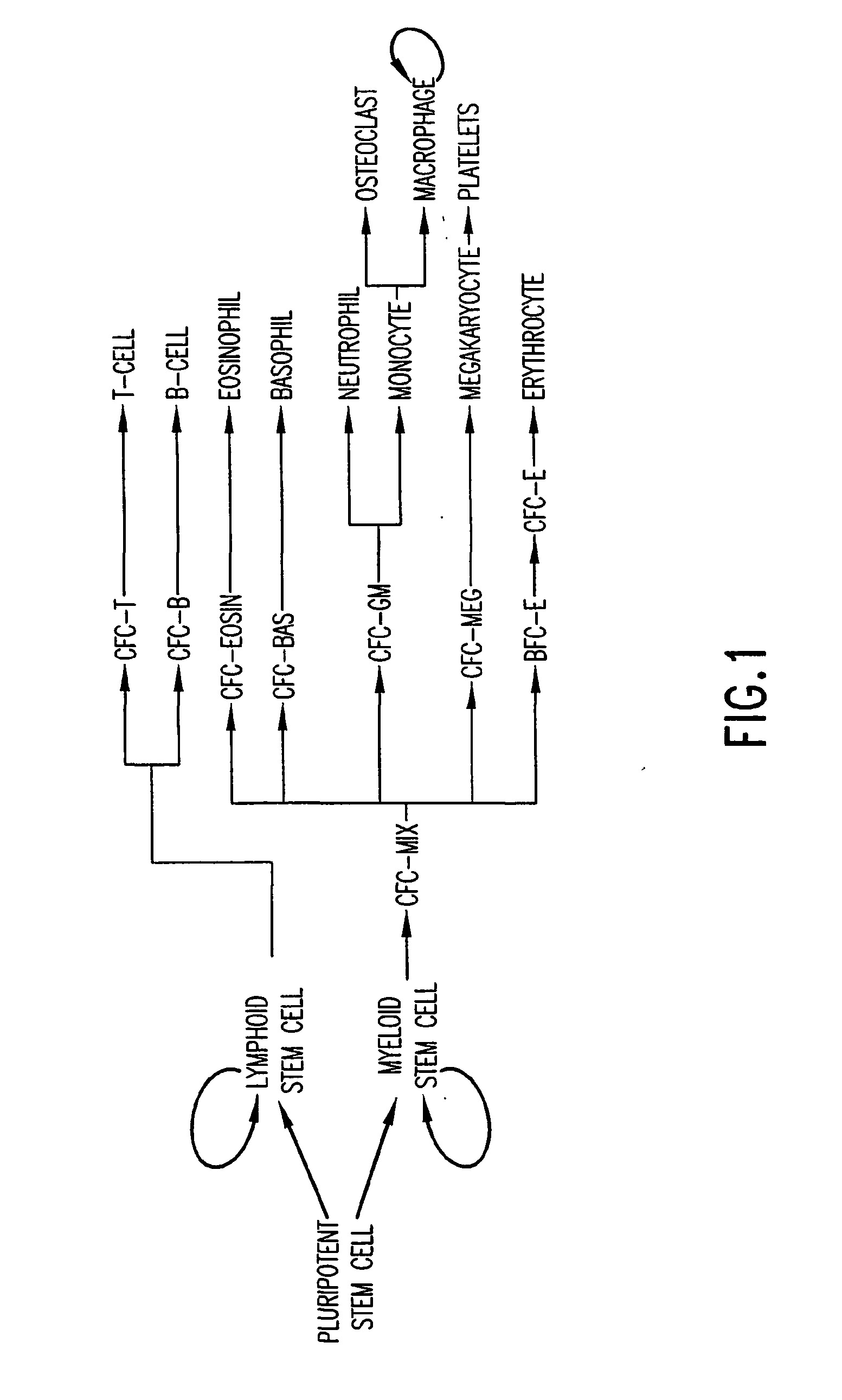
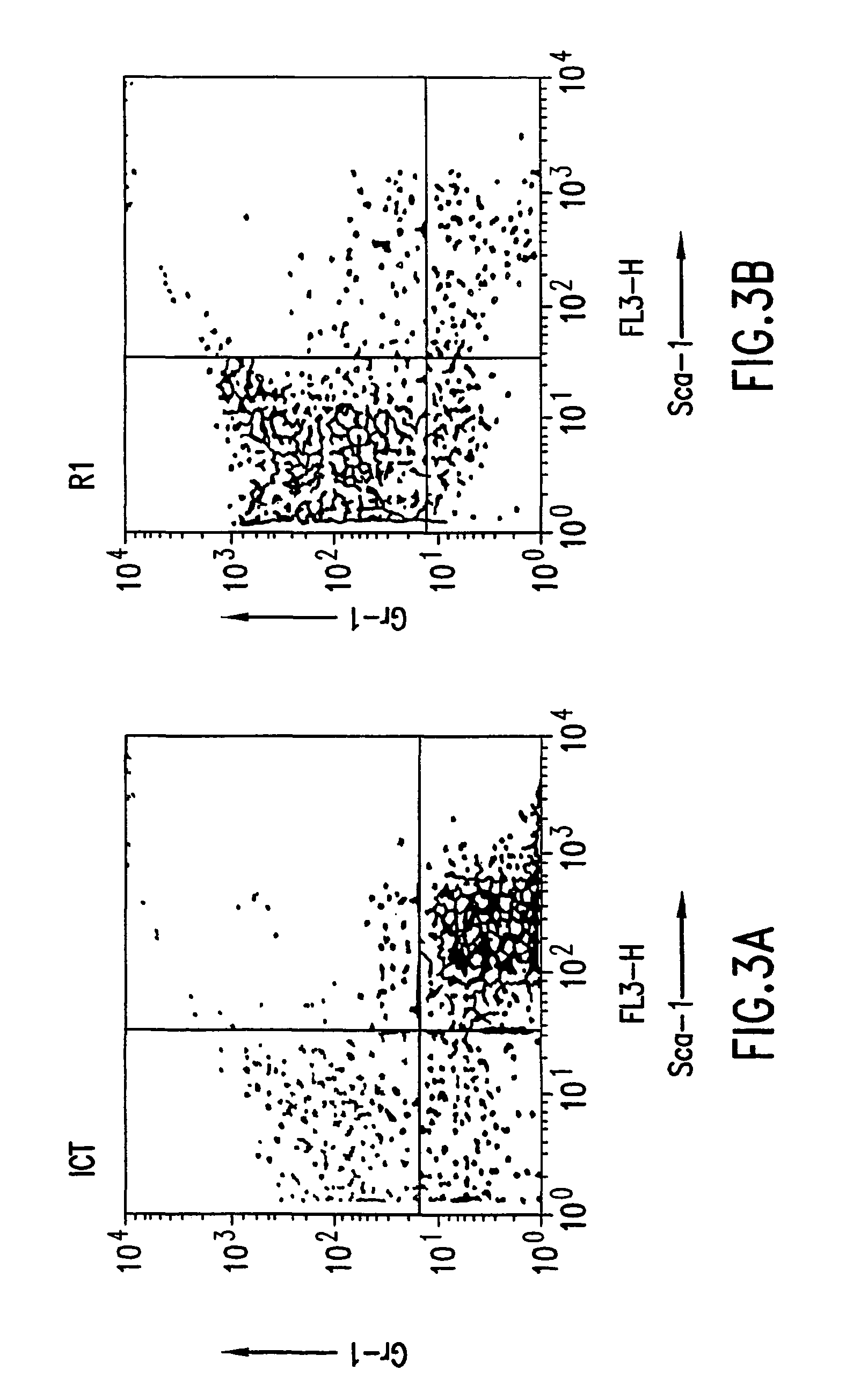
The present invention provides methods for immortalizing precursor cells that are non-terminally differentiated cells such as stem cells, said methods comprising culturing the precursor cells in the presence of a Notch agonist and one or more growth factors that support the proliferation but not differentiation of the non-terminally differentiated cells. The present invention further provides methods to induce the differentiation of immortalized cells, comprising growing the cells in the presence of a Notch agonist and at least one growth factor which supports the differentiation of the cell into a more specialized cell type. The immortalized and / or differentiated cells of the invention can be used to repopulate cell populations that have been diminished, for example as a result of infection or exposure to certain drugs. The invention further provides a cell culture comprising a population of non-terminally differentiated cells immortalized by the methods of the present invention and kits comprising reagents that promote the immortalization of precursor cells. The invention further provides methods for screening for Notch modulators and for identifying genes involved in processes of cellular differentiation.
Owner:FRED HUTCHINSON CANCER CENT
Conditional immortalization of cells
A recombinant, or genetically engineered, mammalian cell, comprises a conditionally-inducible oncogene and an exogenous polynucleotide encoding the catalytic sub-unit of the telomerase complex. The recombinant cells are useful in therapy, to replace lost or damaged cells.
Owner:RENEURON LTD +1
Methods for immortalizing cells
InactiveUS20040067583A1Antibody mimetics/scaffoldsGenetically modified cellsCultured cellSomatic cell
The present invention provides methods for immortalizing precursor cells that are non-terminally differentiated cells such as stem cells, said methods comprising culturing the precursor cells in the presence of a Notch agonist and one or more growth factors that support the proliferation but not differentiation of the non-terminally differentiated cells. The present invention further provides methods to induce the differentiation of immortalized cells, comprising growing the cells in the presence of a Notch agonist and at least one growth factor which supports the differentiation of the cell into a more specialized cell type. The immortalized and / or differentiated cells of the invention can be used to repopulate cell populations that have been diminished, for example as a result of infection or exposure to certain drugs. The invention further provides a cell culture comprising a population of non-terminaly differentiated cells immortalized by the methods of the present invention and kits comprising reagents that promote the immortalization of precursor cells. The invention further provides methods for screening for Notch modulators and for identifying genes involved in processes of cellular differentiation.
Owner:FRED HUTCHINSON CANCER CENT
Intracellular delivery of small molecules, proteins, and nucleic acids
InactiveUS7166692B2Enhance cell viabilityVirus peptidesSaccharide peptide ingredientsProtein transduction domainADAMTS Proteins
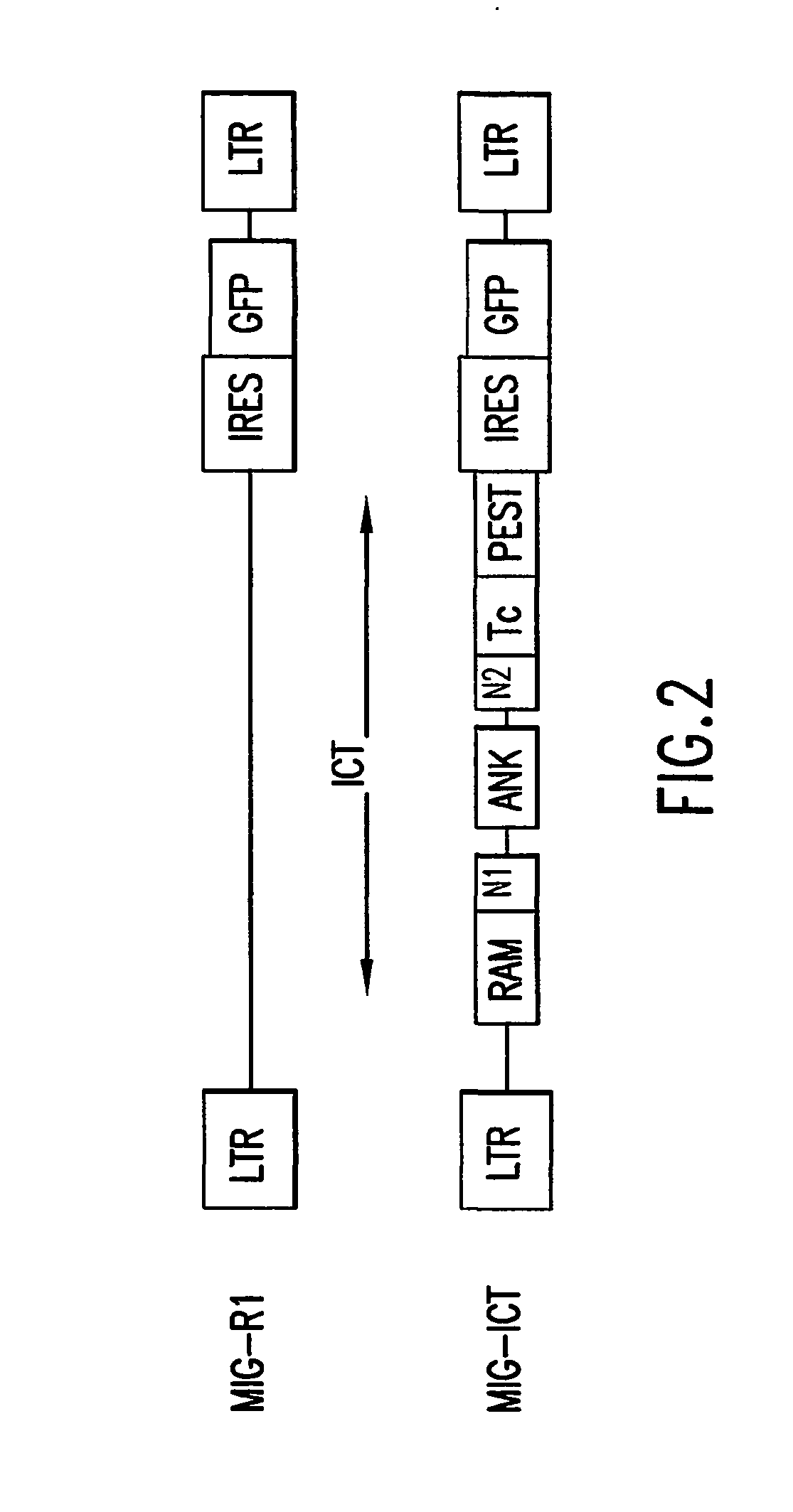
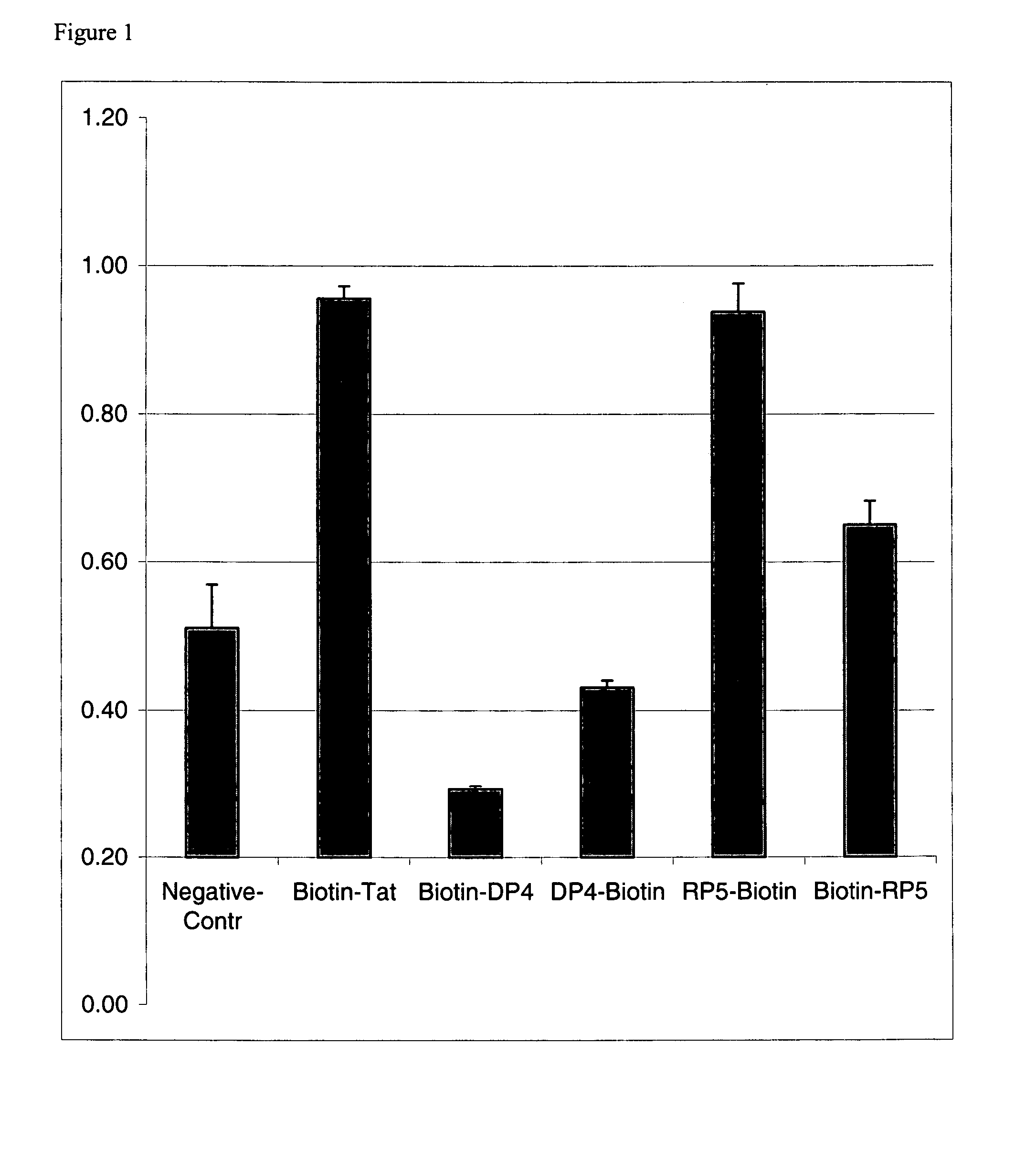
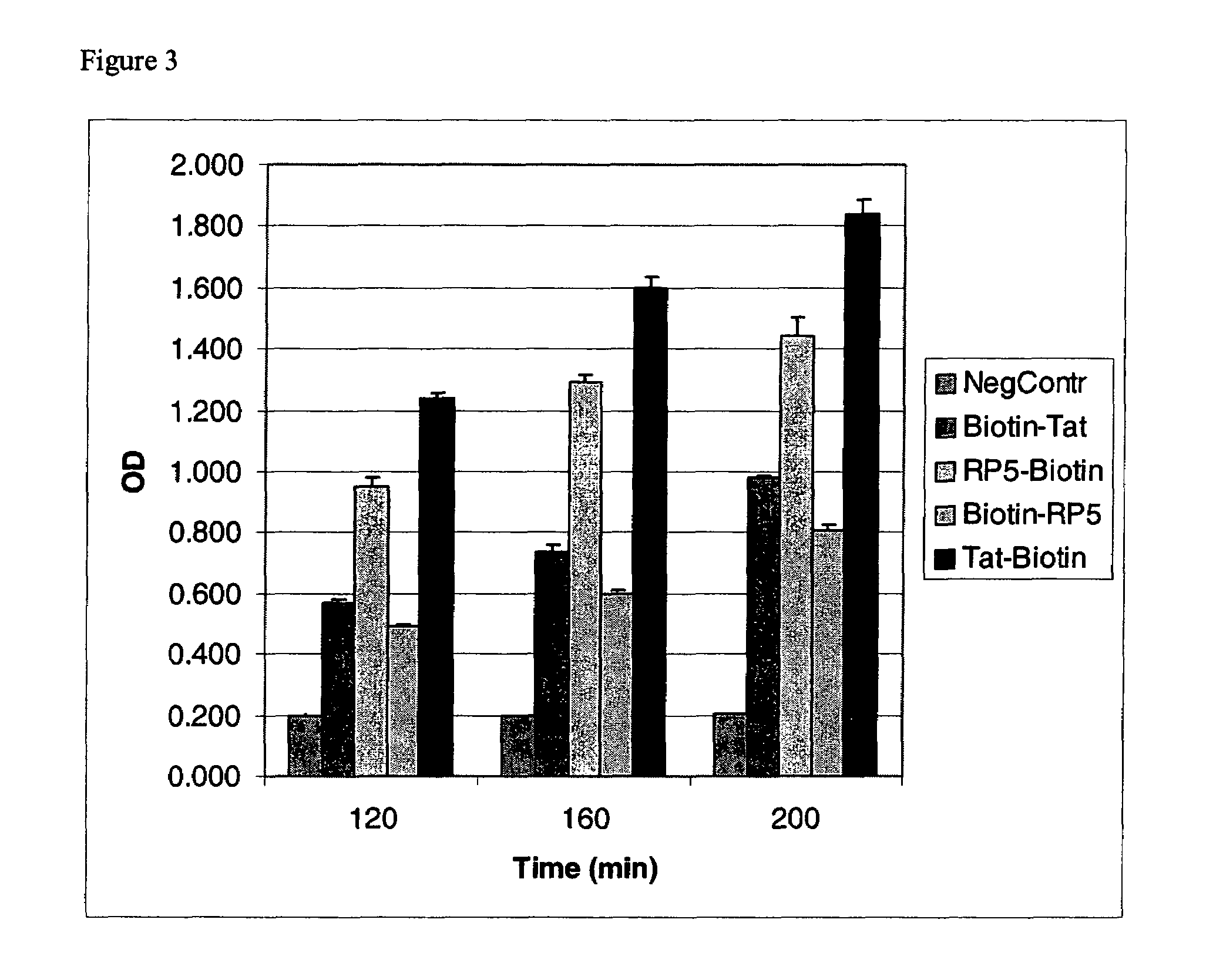
An amino acid sequence Arg-Lys-Met-Leu-Lys-Ser-Thr-Arg-Arg-Gln-Arg-Arg (SEQ ID NO:1) functions as a protein transduction domain (PTD) and is capable of delivering small molecules, proteins, and nucleic acids to an intracellular compartment of a cell. An amino terminal lysine linker improves the efficiency of the PTD. A nuclear localization signal can be used to target the PTD to a cell's nucleus. The PTD can be used in PTD-cargo moiety complexes that can reversibly immortalize cells and increase cell viability in culture.
Owner:LONZA WALKERSVILLE INC
Intracellular delivery of small molecules, proteins, and nucleic acids
InactiveUS7420031B2Enhance cell viabilityUltrasonic/sonic/infrasonic diagnosticsVirusesProtein transduction domainADAMTS Proteins
An amino acid sequence Arg-Lys-Met-Leu-Lys-Ser-Thr-Arg-Arg-Gln-Arg-Arg (SEQ ID NO:1) functions as a protein transduction domain (PTD) and is capable of delivering small molecules, proteins, and nucleic acids to an intracellular compartment of a cell. An amino terminal lysine linker improves the efficiency of the PTD. A nuclear localization signal can be used to target the PTD to a cell's nucleus. The PTD can be used in PTD-cargo moiety complexes that can reversibly immortalize cells and increase cell viability in culture.
Owner:LONZA WALKERSVILLE INC
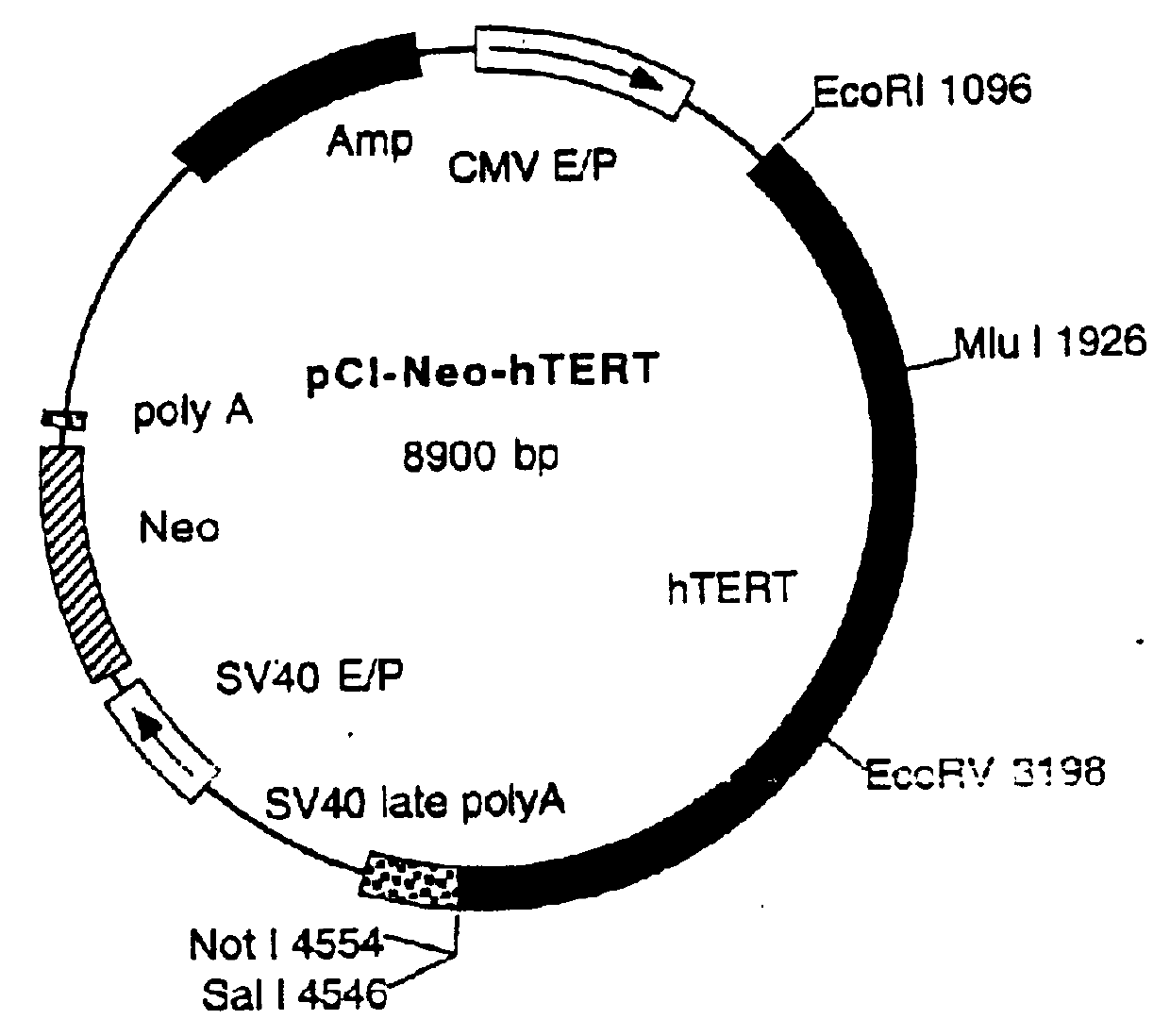
General purpose method for preparing integration type cell immortalization vector
InactiveCN101215577AEfficient immortalization transformationWide range of hostsViruses/bacteriophagesFermentationA-DNAViral vector
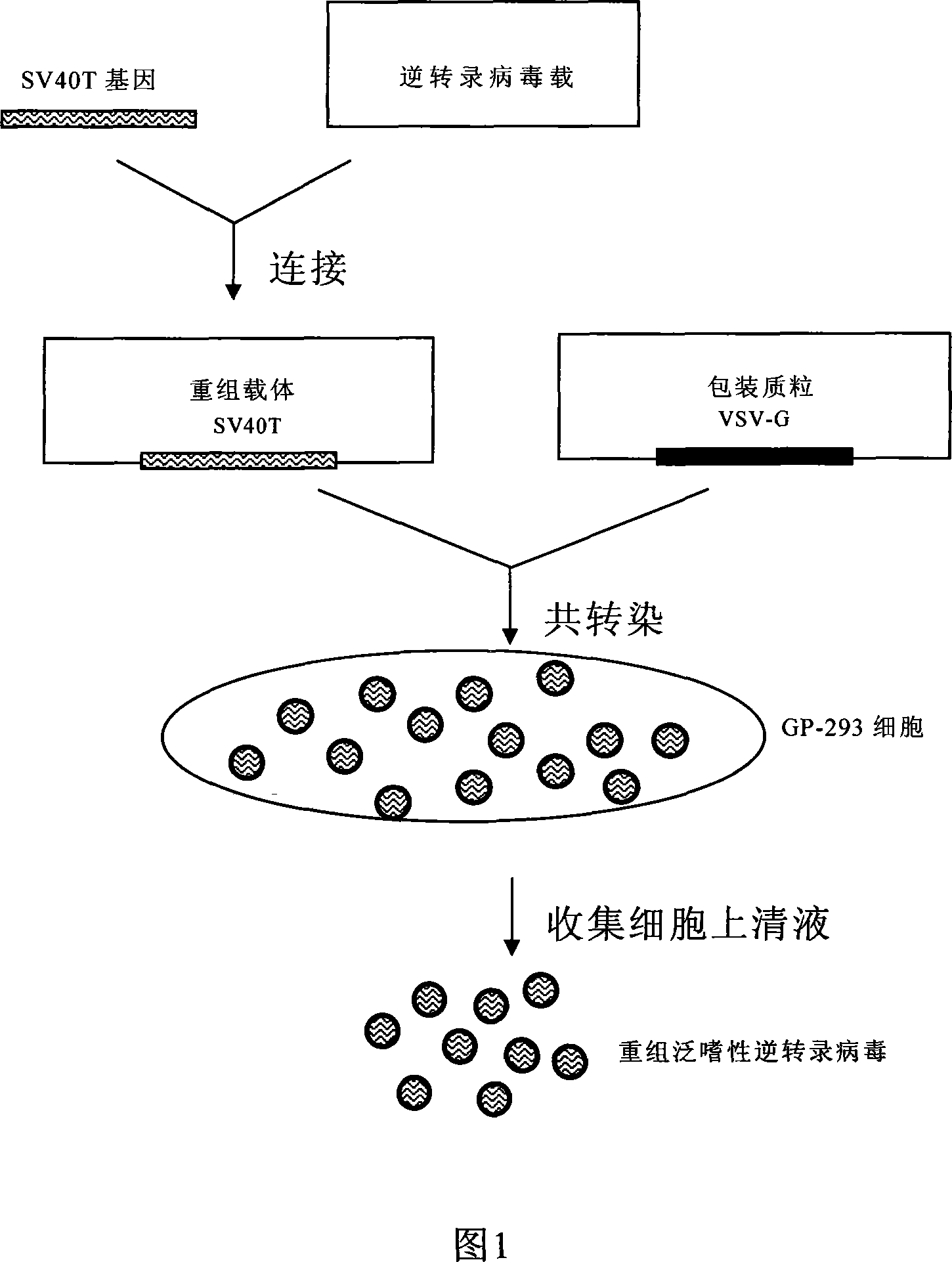
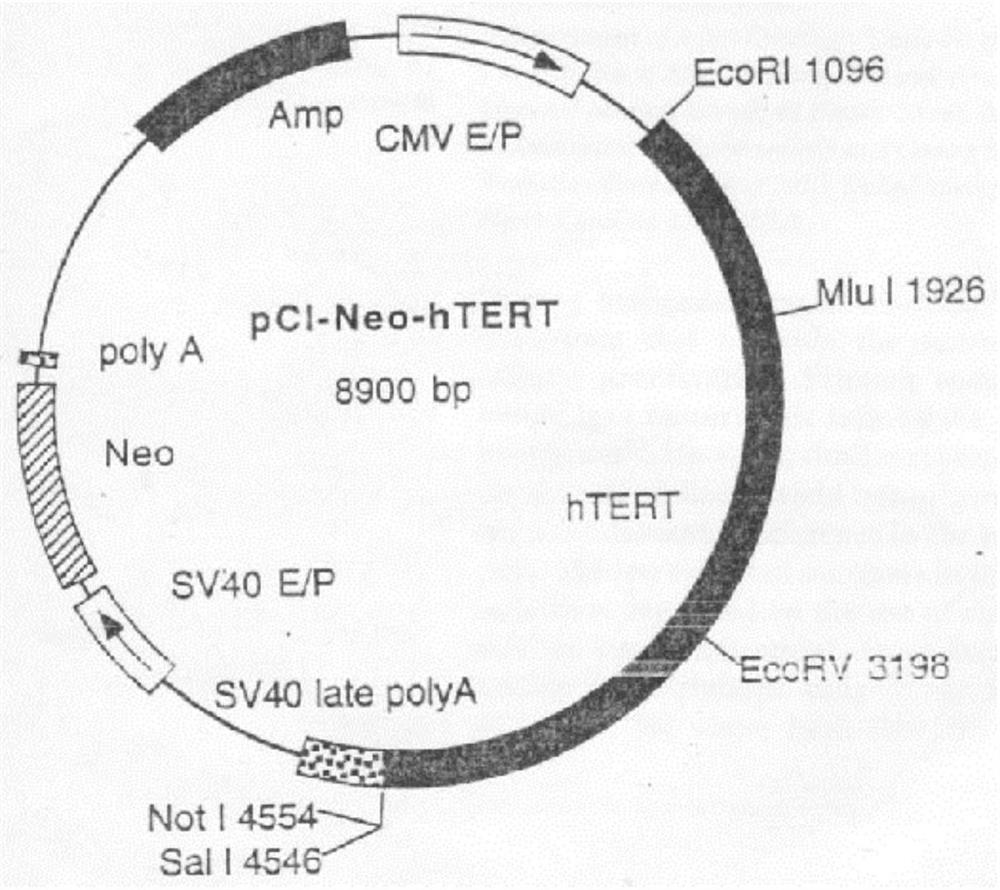
The invention discloses a process for preparing a general integrated type cell immortalized vector. The process for preparation comprises following steps: firstly, directionally inserting a cell immortalized gene-simian virus 40 large T antigens (SV40T) gene with the broad spectrum transforming effect into a retroviral vector through a DNA recombinant technique to construct a recombinant expression vector, then, enabling the recombinant vector and packing plasmid pVSV-G to cotransfect GP-293 cells, and preparing recombinant pantropic reverse transcription virus particles which carry a SV40T gene, wherein the recombinant pantropic reverse transcription virus which is obtained is the general integrated type cell immortalized vector. The immortalized vector can enter into all species of animal cells and can integrate the carried SV40T gene to gene groups of target cells which is in mitotic phase to obtain inheritable and stable expression, and thereby integrated transformation with high efficiency of the target cells can be guaranteed. The invention can provide an effective means for transgenic immortalized establishment and studies of various animal cells.
Owner:OCEAN UNIV OF CHINA
Immortalization of cells including neuronal cells
InactiveUS20090298095A1Improve the level ofCompound screeningApoptosis detectionCell biologyCell immortalization
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Tree shrew hepatocyte immortalized cell line and its construction method and application
InactiveCN106939300AStrong proliferative abilityImprove featuresGenetically modified cellsNucleic acid vectorTumorigenicity TestBiosecurity
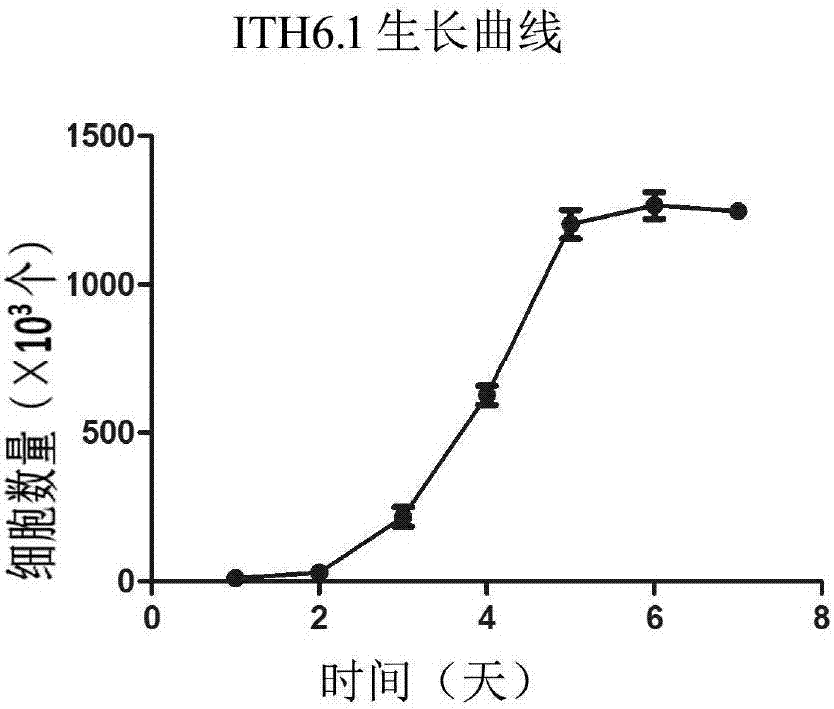
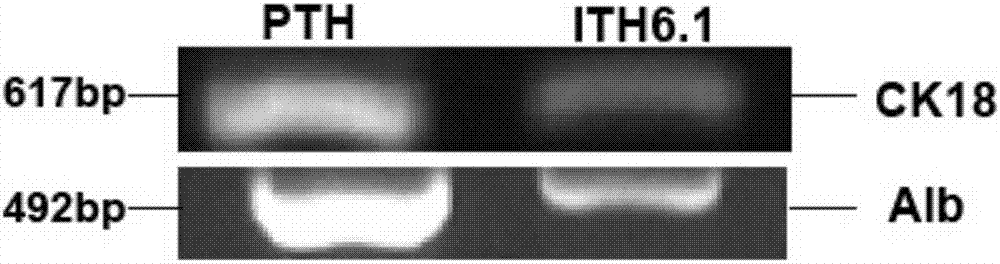
The invention relates to a tree shrew hepatocyte immortalized cell line and its construction method and application and belongs to the technical field of biomedicine. The cell line is named as ITH6.1, is preserved in the China center for type culture collection in the Wuhan University at No. 229, Bayi road, Wuchang District, Wuhan City, Hubei Province and has a preservation number CCTCC NO: C201740. After continuous passage 100 times, the tree shrew hepatocyte immortalized cell line ITH6.1 still keeps a diploid karyotype and has a very strong multiplication capacity so that immortalization is realized really. The cell line has a strong multiplication capacity and realizes protein expression and biochemical index characteristic analysis of ITH6.1. The result shows that the ITH6.1 has typical tree shrew hepatocyte characteristics. The safety test such as a tumorigenicity test also indicates that the cell line has better biosecurity.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
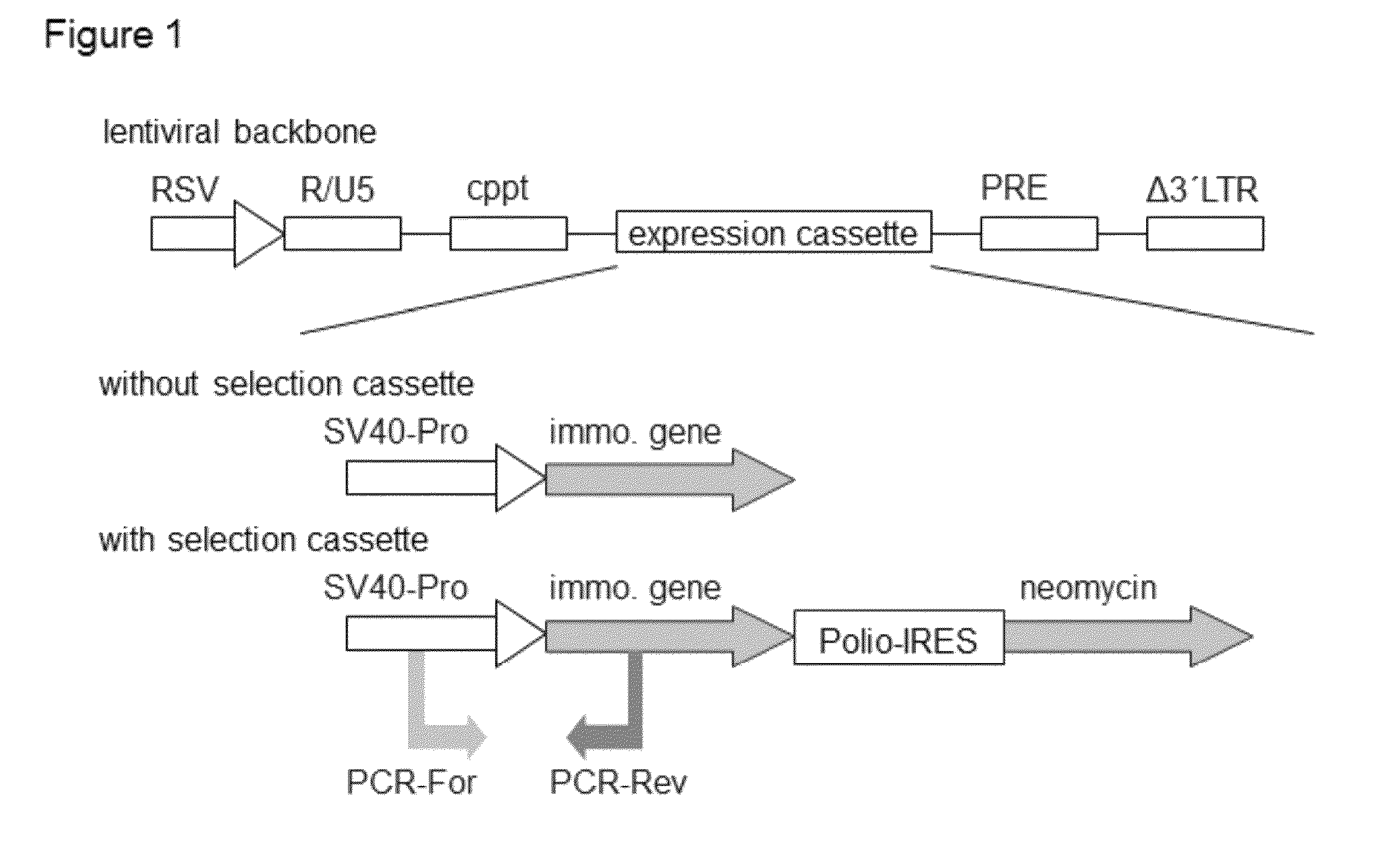
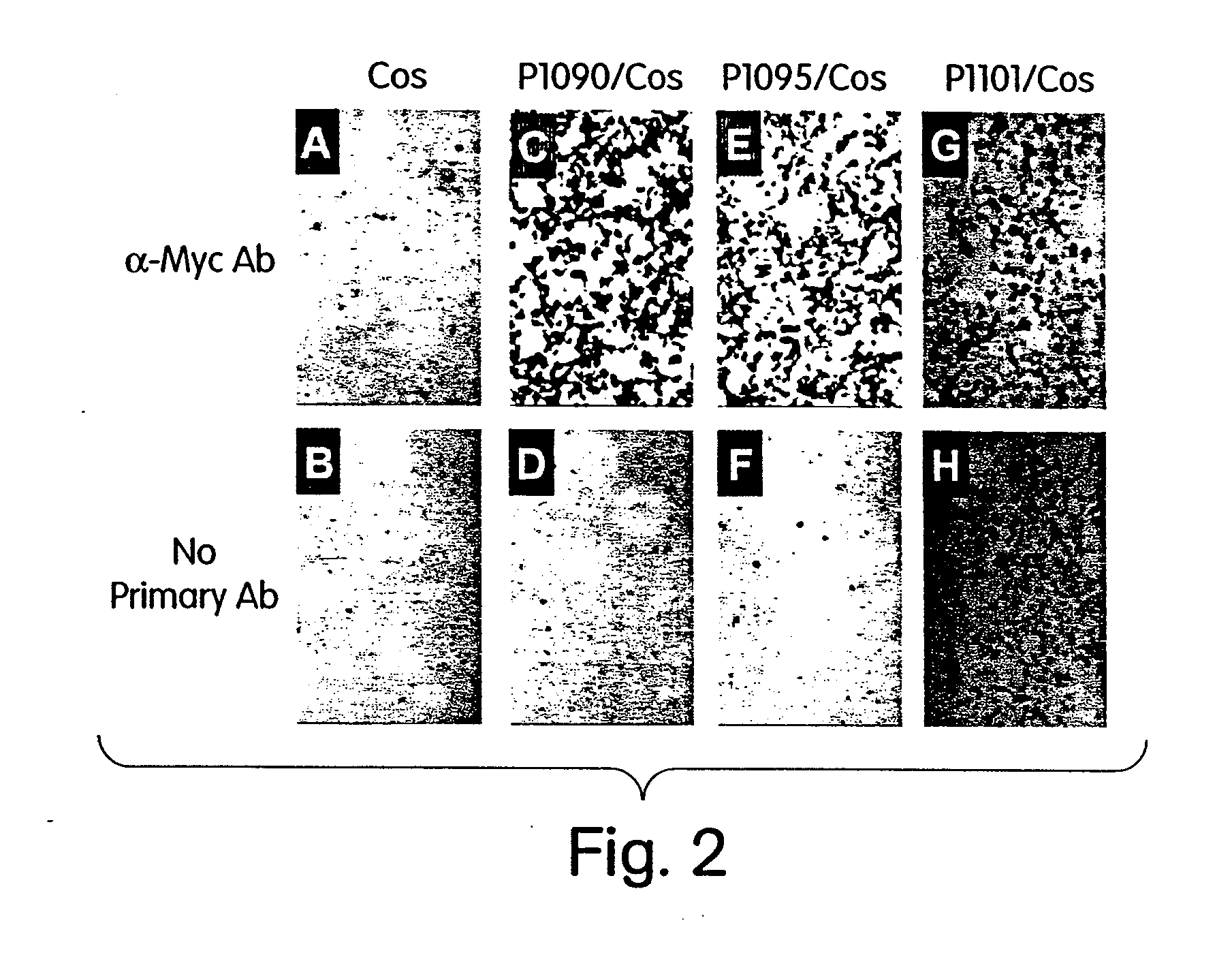
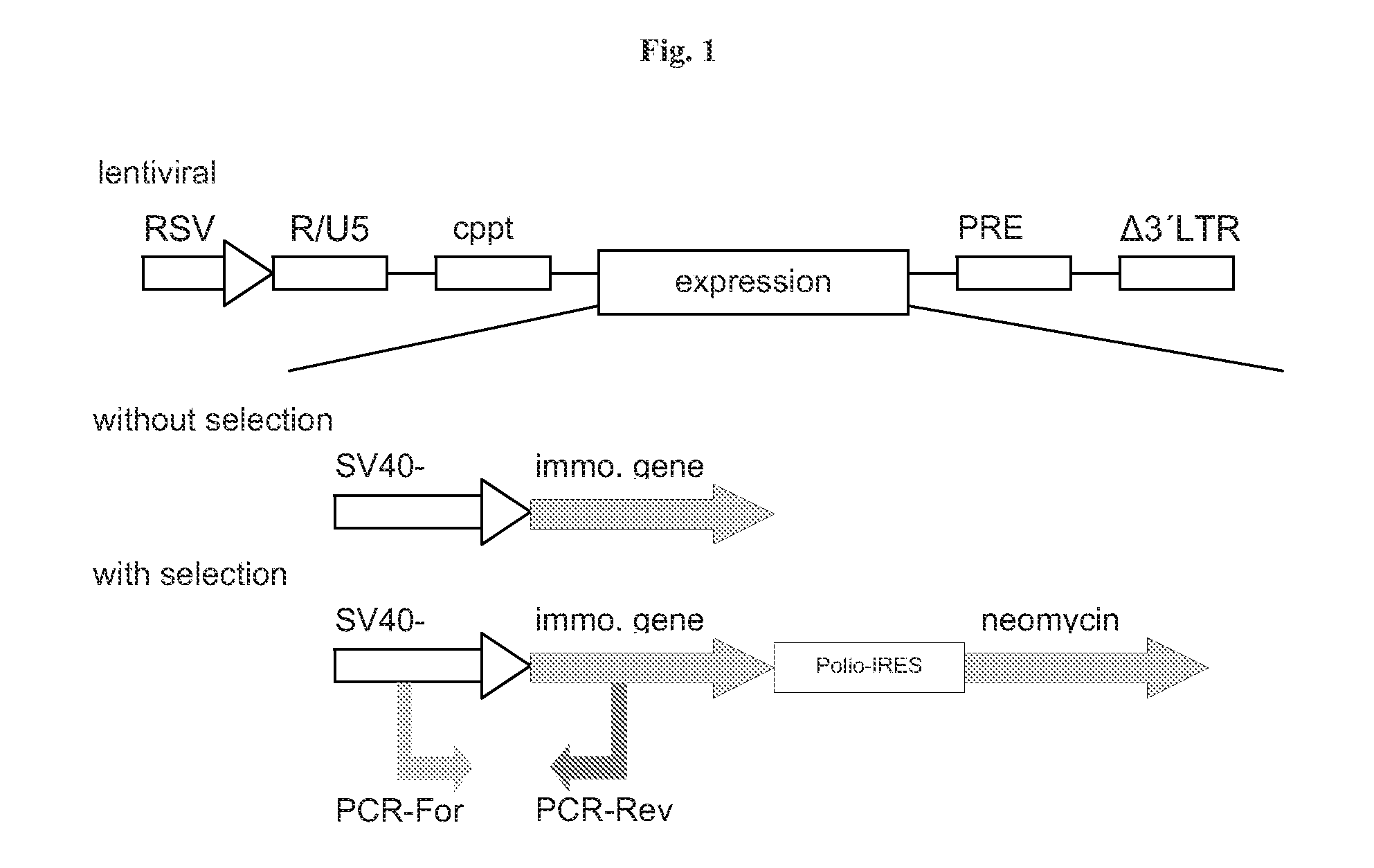
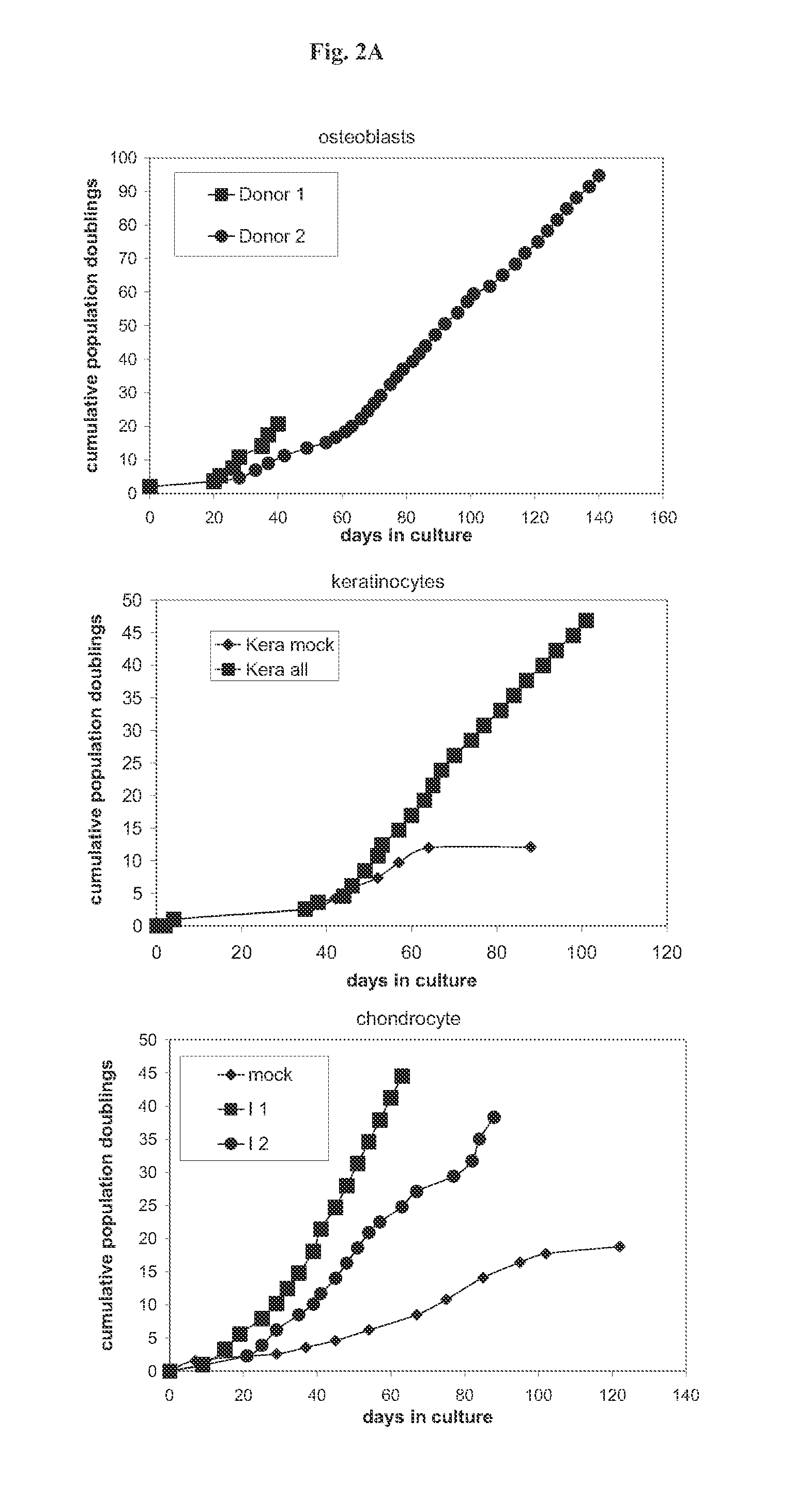
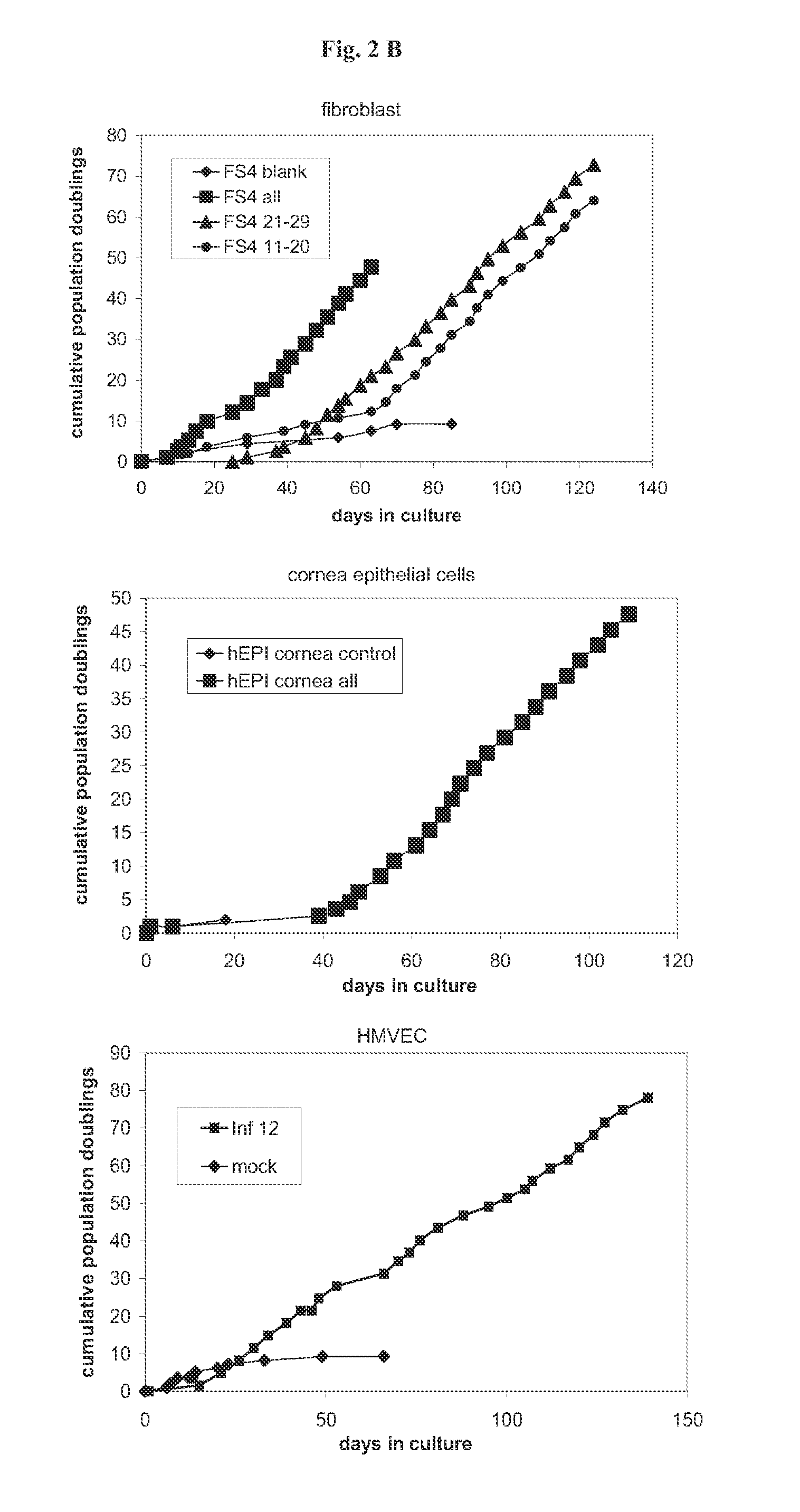
Methods and vectors for cell immortalisation
The present invention relates to a method and to vectors for the immortalisation of cells independent of their type. It further relates to a cell or a cell line produced with the method or the vectors of the invention. The invention also relates to the use of this cell or cell line in in vitro applications and in the treatment of disease.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF
Immortalized goat small intestine epithelial cell line and establishment method thereof
InactiveCN106047819AEasy to operateLow costGastrointestinal cellsGenetically modified cellsSmall intestinePrimary cell
The present invention provides an immortalized goat small intestine epithelial cell line and an establishment method thereof. The method comprises: culturing primary goat small intestine epithelial cells through tissue block, immortalizing the primary-culture goat small intestine epithelial cells, purifying the goat small intestine epithelial cells through a labelling scraping method, and finally establishing the immortalized goat small intestine epithelial cell line. According to the present invention, the addition of any cell growth factors during the immortalized goat small intestine epithelial cell culture process is not required, the operation is convenient and simple, and a lot of cost is saved compared to the primary cell culture; and the method sequentially comprising purifying and cell immortalizing is generally used in the prior art while the labeling method is used to gradually scrap and purify the immortalized goat small intestine epithelial cells, such that the defect that the aging of the goat small intestine epithelial cells due to the prolonged culture time finally causes the cell immortalization failure in the prior art can be avoided.
Owner:YANGZHOU UNIV
Lamb testis support cell immortalized cell line and establishment method and application thereof
ActiveCN109837250AExtend your lifeEfficient ProliferationMicroorganism based processesViruses/bacteriophagesCell divisionTesticle
The invention discloses a lamb testis support cell immortalized cell line and an establishment method and application thereof. The lamb testis support cell immortalized cell line is named a lamb testis support cell immortalized cell line hTERT-LSC with a collection number CCTCC NO: C2018202. Tests find that the hTERT-LSC cell line can realize continuous passage for more than 60 generations, and the growth and proliferation characteristics are not changed. The cell line is relatively sensitive to inoculation of the sheep pox virus, can efficiently proliferate the sheep pox virus, and can ensurethe uniformity and stability of the virus. The titer of the sheep pox virus proliferated by the cell line is equivalent to that of the lamb testis support cell LSC, and the cell division rate is higher than that of the LSC and can reach 1:3. Furthermore, by utilizing the immortalized cell line for preparing a vaccine, the production process is simplified, the production cycle is shortened, the production cost is reduced, and simultaneously, the quality stability of a prepared sheep pox virus vaccine is also ensured. Therefore, the invention provides a new technical means for large-scale production of the sheep pox virus vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Transiently immortalized cells for use in gene therapy
InactiveUS20080064102A1Efficient and rapid reversionEfficient transportHydrolasesAntibody mimetics/scaffoldsHuman cellBiological activation
The invention provides methods and compositions for expanding cells that are not abundant or are difficult to obtain in pure form in culture, are in short supply (e.g., human cells), or have brief lifetimes in culture, using fusion polypeptide. The fusion polypeptide has a first region having the transport function of herpesviral VP22 protein or human immunodeficiency virus (HIV) TAT protein, and a second region with a polypeptide having cell immortalization activity, a polypeptide having telomerase-specific activity, or a polypeptide having telomerase gene activation activity. The resulting cells of the invention are suitable for use in cell therapy.
Owner:HEART BIOSYST
Methods and vectors for cell immortalisation
ActiveUS20130273550A1Facilitate cell cycle progressionPromote activationSenses disorderVirusesDiseaseCell biology
The present invention relates to a method and to vectors for the immortalisation of cells independent of their type. It further relates to a cell or a cell line produced with the method or the vectors of the invention. The invention also relates to the use of this cell or cell line in in vitro applications and in the treatment of disease.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF
Newborn sertoli-cell immortalization cell line and establishing method and application thereof
ActiveCN109735499AExtend your lifeThe characteristics of growth and proliferation are unchangedMicroorganism based processesViruses/bacteriophagesTiterSertoli cell
The invention discloses a newborn sertoli-cell immortalization cell line and an establishing method and application thereof. The newborn sertoli-cell immortalization cell line is named as the newbornsertoli-cell immortalization cell line hTERT-NBSC, and the collection number of the hTERT-NBSC is CCTCC NO:C2018201. Tests find that the hTERT-NBSC can continuously pass 65 generations or above, and the growth and proliferation characteristic is kept constant. The cell line is sensitive to inoculation of ORFV, the ORFV virus can be efficiently proliferated, and the uniformity and the stability ofthe virus can be guaranteed. The titer of the ORFV virus proliferated by the cell line is equivalent to that of the newborn sertoli cell, the cell sorting rate of the ORFV virus is higher than that ofthe NBSC, and the cell sorting rate can be 1:3. Meanwhile, vaccine is prepared with the passage cell line, the production technology is simplified, the production cycle is shortened, the production cost is reduced, and the stable quality of the contagious ovine ecthyma vaccine prepared with the method is also guaranteed. Therefore, through the newborn sertoli-cell immortalization cell line and the establishing method and application thereof, a new technological mean is provided for large-scale production of the contagious ovine ecthyma vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
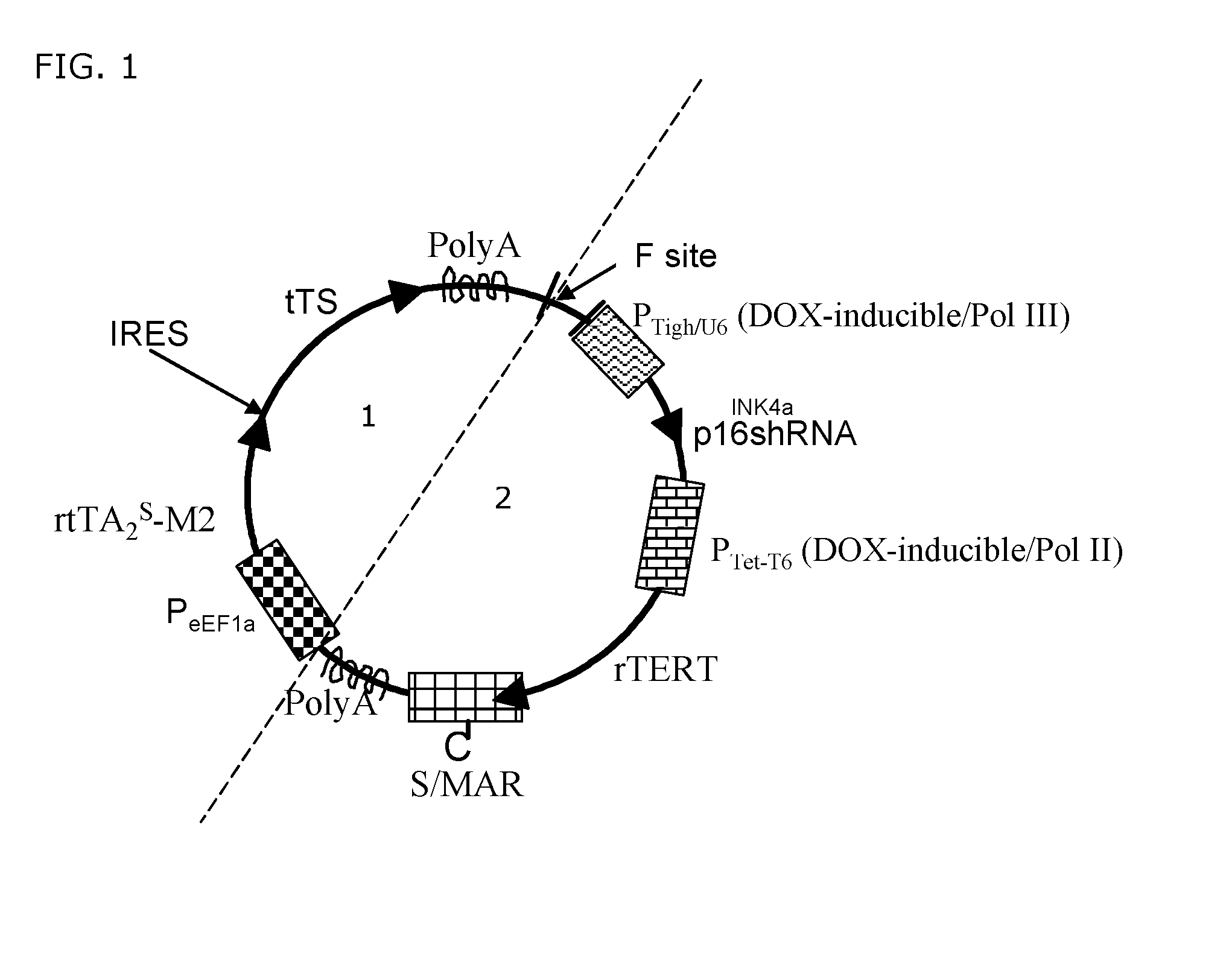
Transgenic system for reversibly immortalizing mammalian quiescent cells
ActiveUS20140199766A1Genetically modified cellsArtificial cell constructsMammalTranscriptional regulation
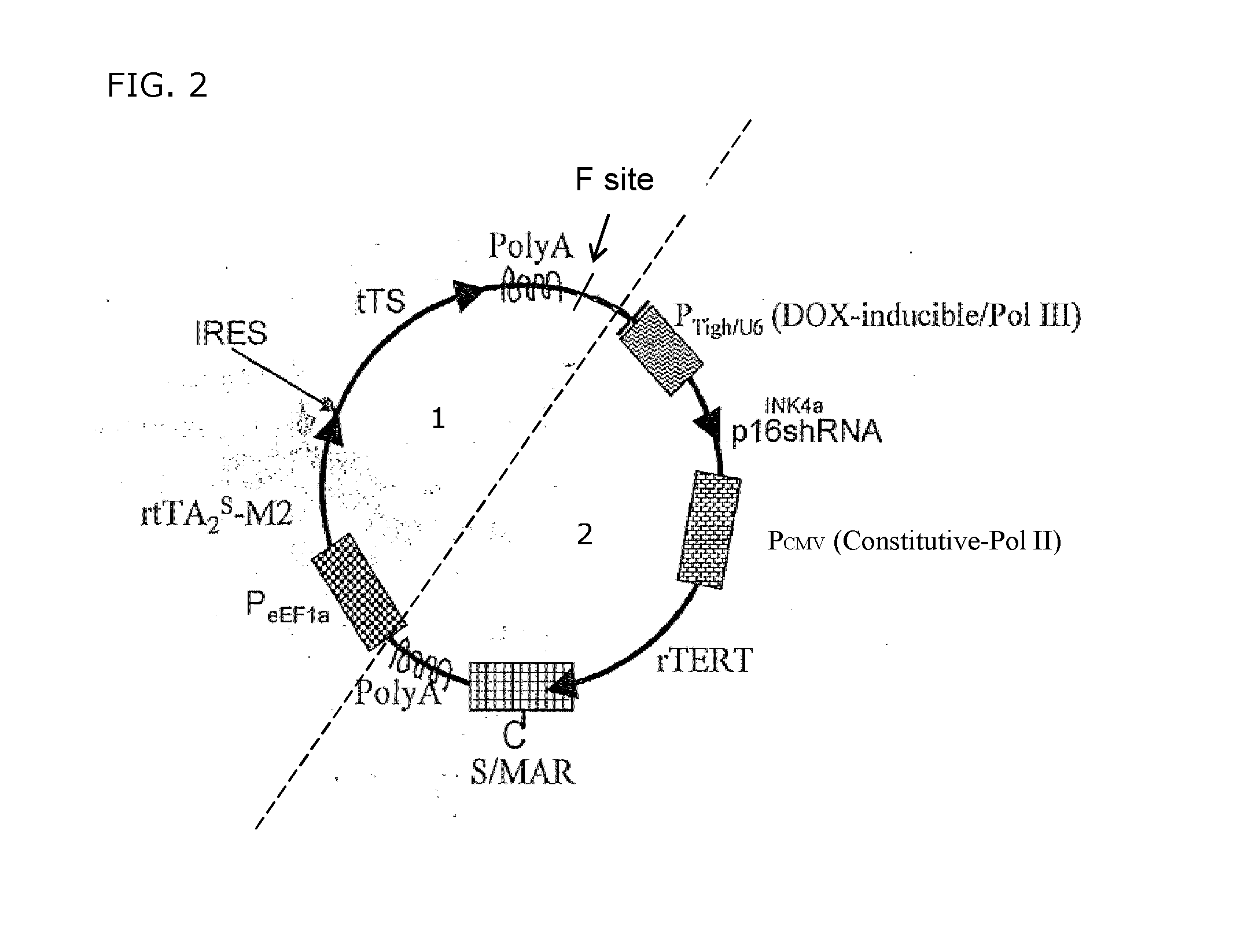
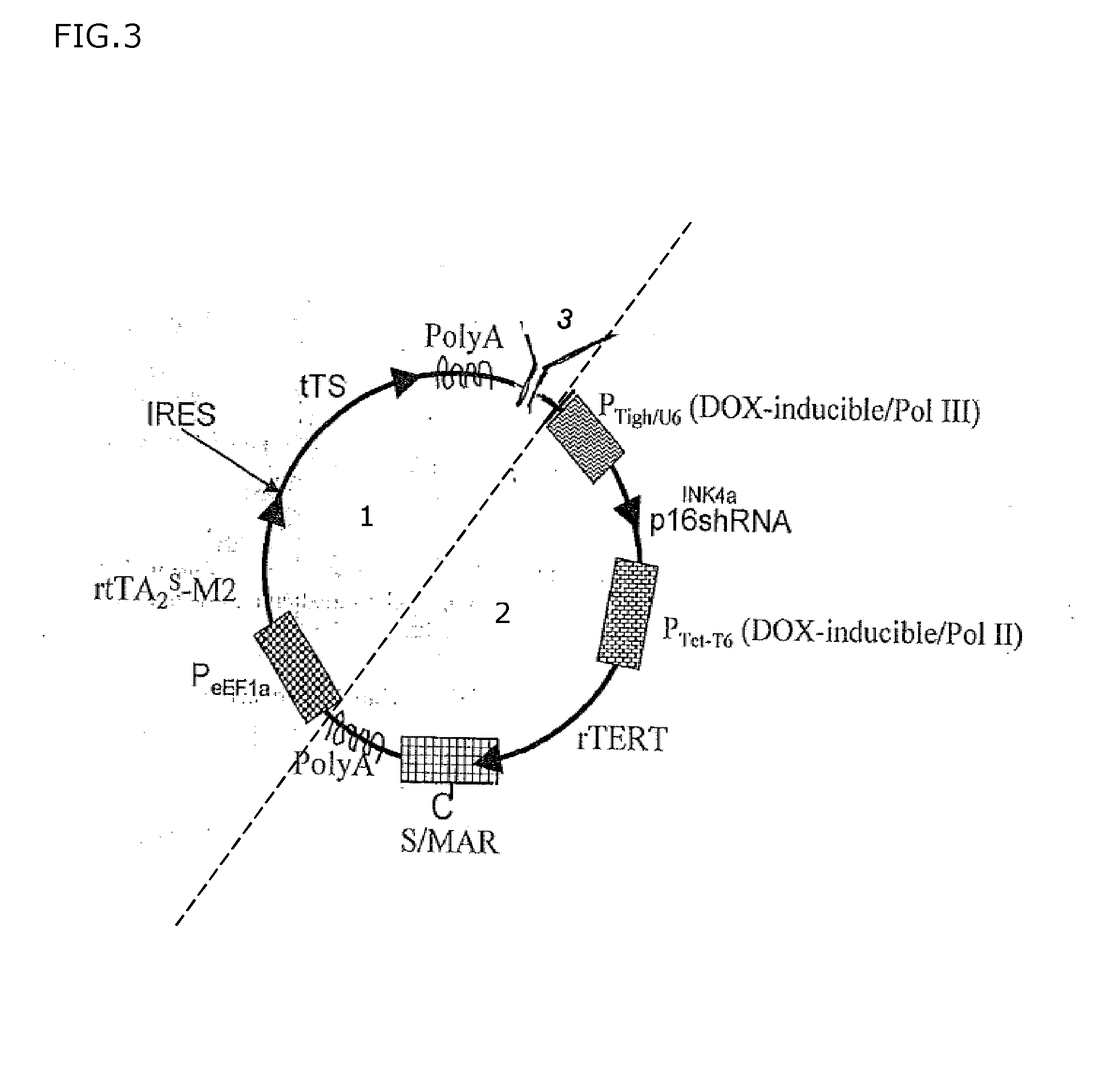
A transgene for endowing human quiescent cells from primary cultures with exogenously inducible telomere length homeostasis and cell cycle traverse is described. The transgene includes at least three promoter sequences; a G1-specific RNA interference sequence which is operably linked downstream of a first promoter sequence; a telomere length maintenance gene sequence which is operably linked downstream to a second promoter sequence; a Scaffold / Matrix attachment region (S / MAR) element positioned downstream in frame with said telomere length maintenance gene sequence and an exogenously-inducible transcriptional regulation system. The transgene is useful to immortalize human quiescent cells from primary cultures.
Owner:CILBIOTECH
Cancer stem cell immortalization
The present invention relates to the preparation and use of immortalized cancer stem cells. The immortalized cancer stem cells of the invention may be used in assays to identify anti-cancer compounds as well as molecules critical to carcinogenesis. Further, cancer stem cells may be harvested from a patient and used, according to the invention, to select agents more likely to be effective in treating the cancer of that particular patient.
Owner:UNIVERSITY OF PITTSBURGH
Construction method of immortalized feeder layer cell strain, immortalized feeder layer cell strain and application
PendingCN112941033AExtensive Cell ResourcesCompound screeningApoptosis detectionCell strainTumor cells
The invention discloses a construction method of an immortalized feeder layer cell strain, the immortalized feeder layer cell strain and application of the immortalized feeder layer cell strain. At least two immortalized genes are selected to be infected into feeder layer cells, and the two immortalized genes achieve a synergistic promotion effect on feeder layer cell immortalization through different cell immortalization promotion ways, and the immortalized feeder layer cell strain is obtained, so that the blank of a feeder layer cell immortalization method is filled, and wide cell resources are provided for evaluating the effect of treating tumors by drugs or controlling the proliferation of tumor cells and determining the proliferation or aging mechanism of the cells.
Owner:SHENZHEN PEOPLES HOSPITAL
Method for detecting activity of telomerase
PendingCN113774112AReduce usageSolve complexityMicrobiological testing/measurementBiological material analysisFluoProbesCancer cell
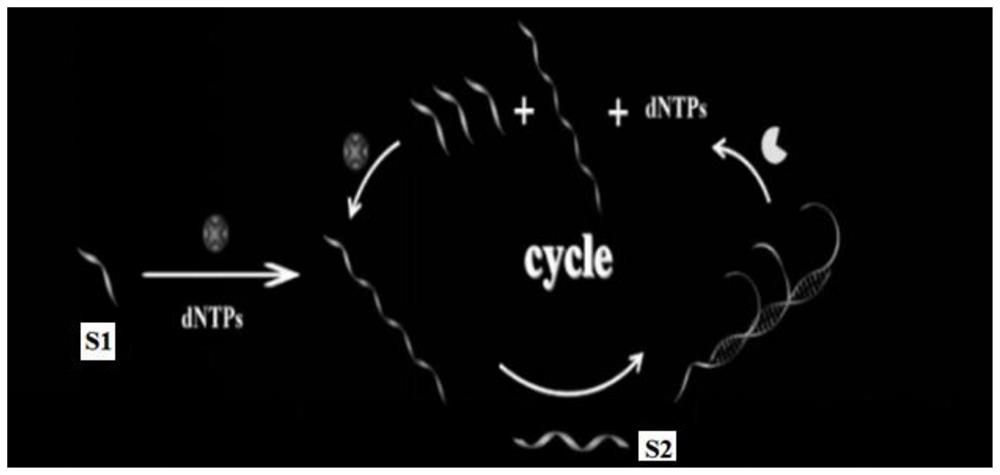
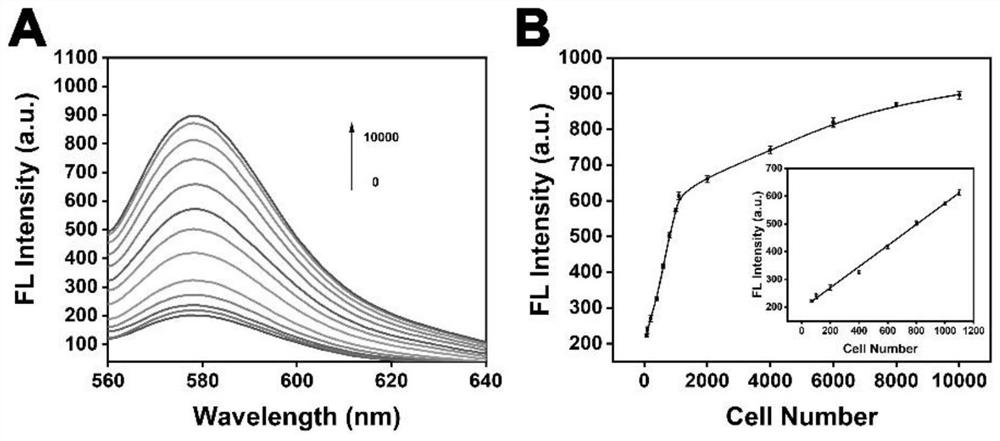
Telomerase can maintain the length of telomere by triggering amplification reaction, thereby causing cell immortalization and carcinogenesis. Research shows that telomerase is overexpressed in almost all cancer cells, so that telomerase becomes one of important biomarkers. Therefore, the development of a technology for sensitively detecting telomerase is of great significance for early diagnosis and treatment of tumors. The invention designs and develops a fluorescent probe for detecting the activity of telomerase based on blocking materials S1 and S2. A telomerase primer S1 capable of triggering repeated amplification reaction of telomerase and S2 capable of undergoing multiple hybridization reaction with an amplification product are adopted as the blocking materials of a porous nano-carrier to simulate and construct an intelligent lock, so that high-specificity and ultra-sensitive detection of the activity of telomerase in cancer cells is realized, the defects of low detection sensitivity, large sample demand, tedious steps, high cost and the like of a traditional method are overcome, and a new strategy and method are provided for living body and cell detection and in-situ imaging.
Owner:QINGDAO UNIV OF SCI & TECH
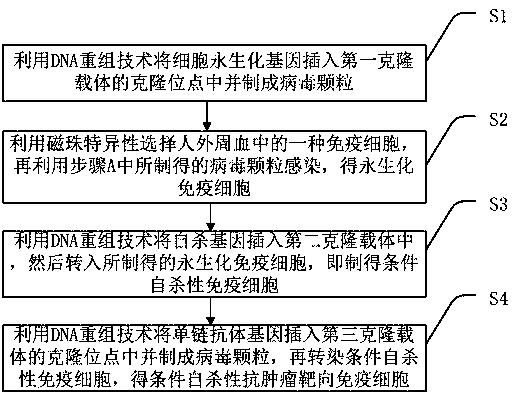
Conditional suicidal anti-tumor targeting immune cell and preparation method thereof
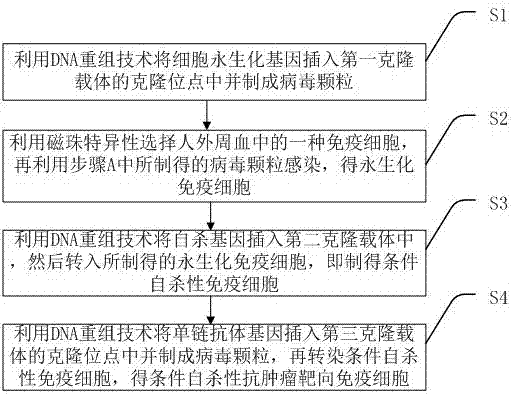
InactiveCN106867969AAvoid potential side effectsResolve Healing EffectsGenetically modified cellsBlood/immune system cellsTumor targetHuman body
The invention discloses a conditional suicidal anti-tumor targeting immune cell and a preparation method thereof. The preparation method comprises the following steps of A, using a DNA (deoxyribonucleic acid) reconstruction technique to insert a cell immortalized gene into a cloning site of a first cloning carrier, and preparing a virus particle; B, utilizing magnetic beads to specifically select one type of immune cell from human peripheral blood, and utilizing the virus particle prepared in step A to infect, so as to obtain an immobilized immune cell; C, utilizing the DNA reconstruction technique to insert a suicidal gene into a second cloning carrier, and transferring into the prepared immobilized immune cell, so as to obtain a conditional suicidal immune cell; D, utilizing the DNA reconstruction technique to insert a single-chain antibody gene into a cloning site of a third cloning carrier, and preparing into a virus particle; transfecting the conditional suicidal immune cell, so as to obtain the conditional suicidal anti-tumor targeting immune cell. The preparation method can solve the problems of great side effect, failure to widely apply in clinic, short surviving time in human body, higher cost and poor controllability in the tumor cell targeting treatment process of the prior art.
Owner:苏州博棠再生医学科技有限公司
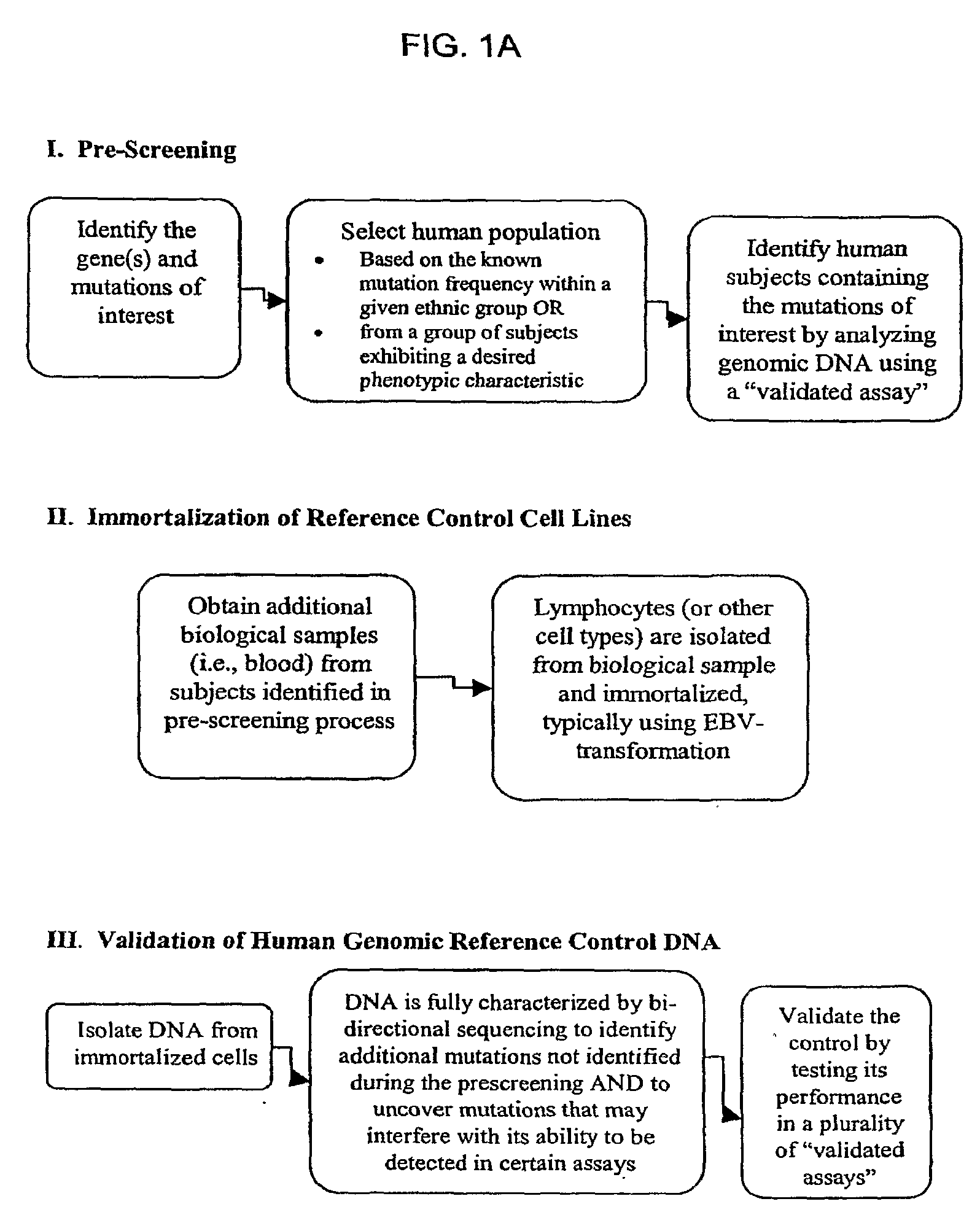
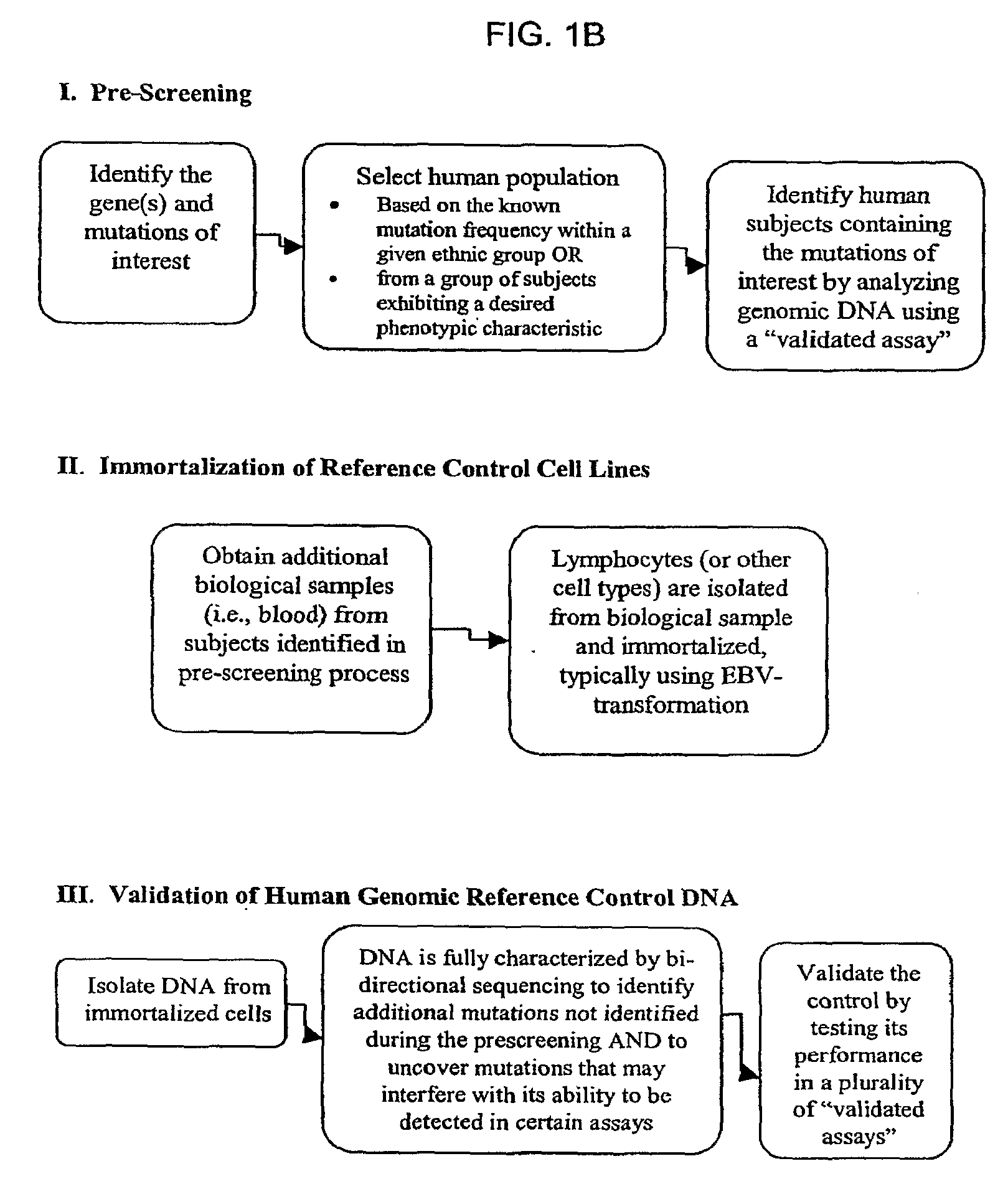
Method for Generating Reference Controls for Pharmacogenomic Testing
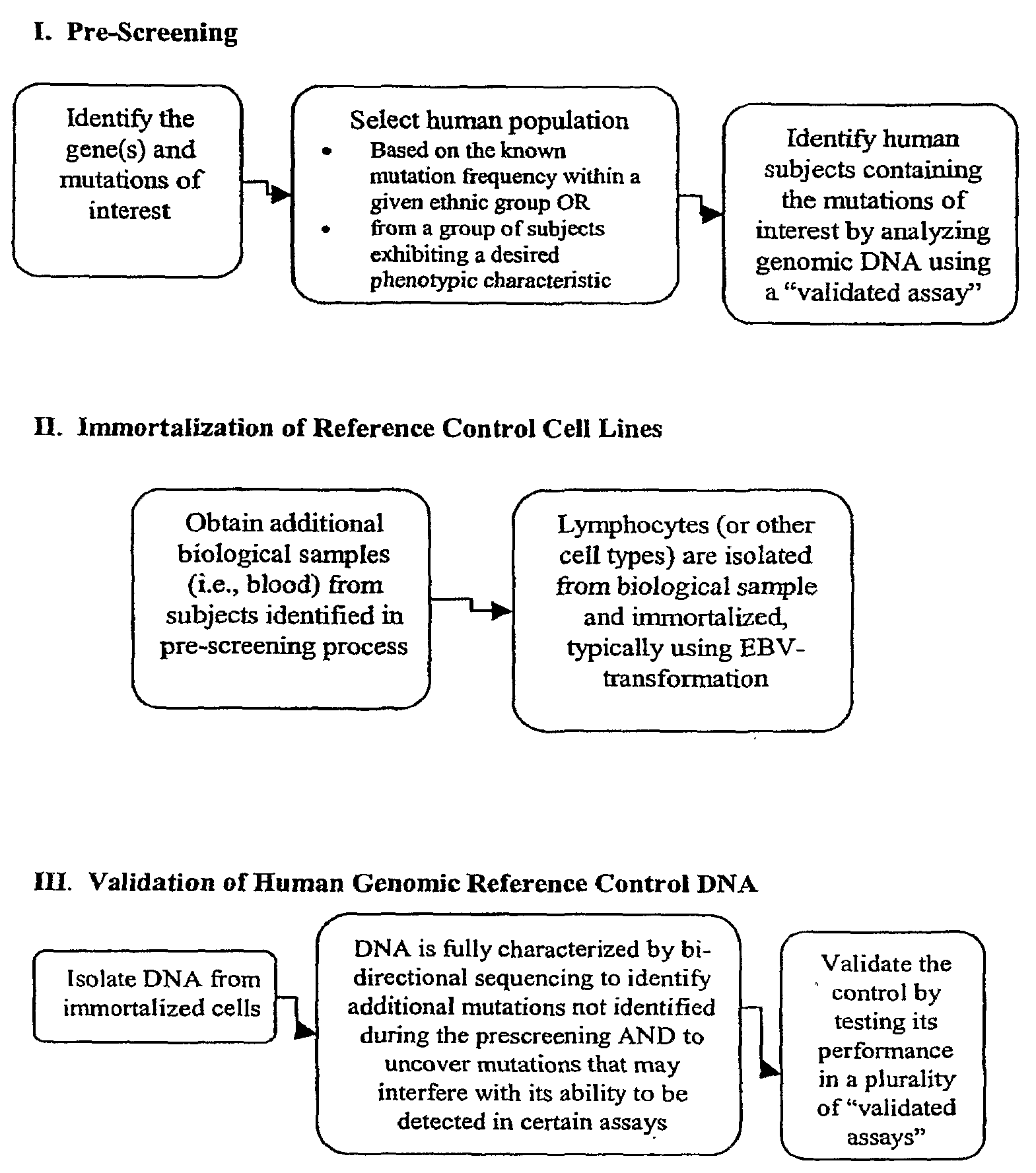
InactiveUS20090197945A1Accurate identificationAccurate doseBiocideNervous disorderGenomicsGenome human
Reference controls for use with pharmacogenomic testing, and methods for their identification, preparation, and use, are disclosed. The reference controls can confirm that pharmacogenomic testing correctly identifies individuals that do or do not have the mutation of interest, in both clinical trial and patient treatment settings. The reference controls can be selected to include one or more mutations to be identified, and prescreened to confirm that they bind to one or more of the primers used in the pharmacogenomic testing. The reference controls are human genomic DNA that includes certain identified polymorphisms (mutations) of interest, ideally derived from individuals, pre-selected and optionally properly consented, which have one or more of the polymorphism(s) of interest. The reference controls can be prepared by targeted pre-screening of human patients, by examining the genotype or genetic profile of the patients, isolating cells with the desired mutation, optionally immortalizing the cells, and obtaining DNA from the cells. The prescreening of prospective donors can be targeted based on any of a number of factors, such as genes of interest, mutations within the genes of interest, and membership in a specific ethnic or disease state population. The genomic DNA can be pre-screened for its ability to be detected, using a standard pharmacogenomic test, as including a specific mutation. Examples of mutations of interest include those present in a Phase I or Phase II metabolic enzyme such as CYP2D6, CYP2C19, CYP2C9, CYP2C8, and CYP3A5, CYP3A4, CYP2A6, CYP2B6, UGT1A1, DPD, ERCC1, MDR1, ADH2, NAT1 and NAT2 or any other metabolic or disease gene.
Owner:CATALYST ASSETS LLC
Conditional suicide anti-tumor targeted immune cell and preparation method thereof
InactiveCN106867969BAvoid potential side effectsGenetically modified cellsBlood/immune system cellsTumor targetSingle-Chain Antibodies
The invention discloses a conditional suicide anti-tumor targeted immune cell and a preparation method thereof, which includes the steps of: A. Using DNA recombinant technology to insert the cell immortalization gene into the cloning site of the first cloning vector and prepare virus particles; B. . Use magnetic beads to specifically select an immune cell in human peripheral blood, and then use the virus particles prepared in step A to infect to obtain immortalized immune cells; C. Use DNA recombinant technology to insert the suicide gene into the second cloning vector. into the prepared immortalized immune cells to obtain conditional suicide immune cells; D. Use DNA recombinant technology to insert the single-chain antibody gene into the cloning site of the third cloning vector and make virus particles, Conditioned suicidal immune cells are then transfected to obtain conditional suicidal anti-tumor targeting immune cells. It solves the problems in the existing technology of tumor cell targeted therapy with large side effects, inability to be widely used clinically, short in vivo survival time, high cost, and poor controllability.
Owner:苏州博棠再生医学科技有限公司
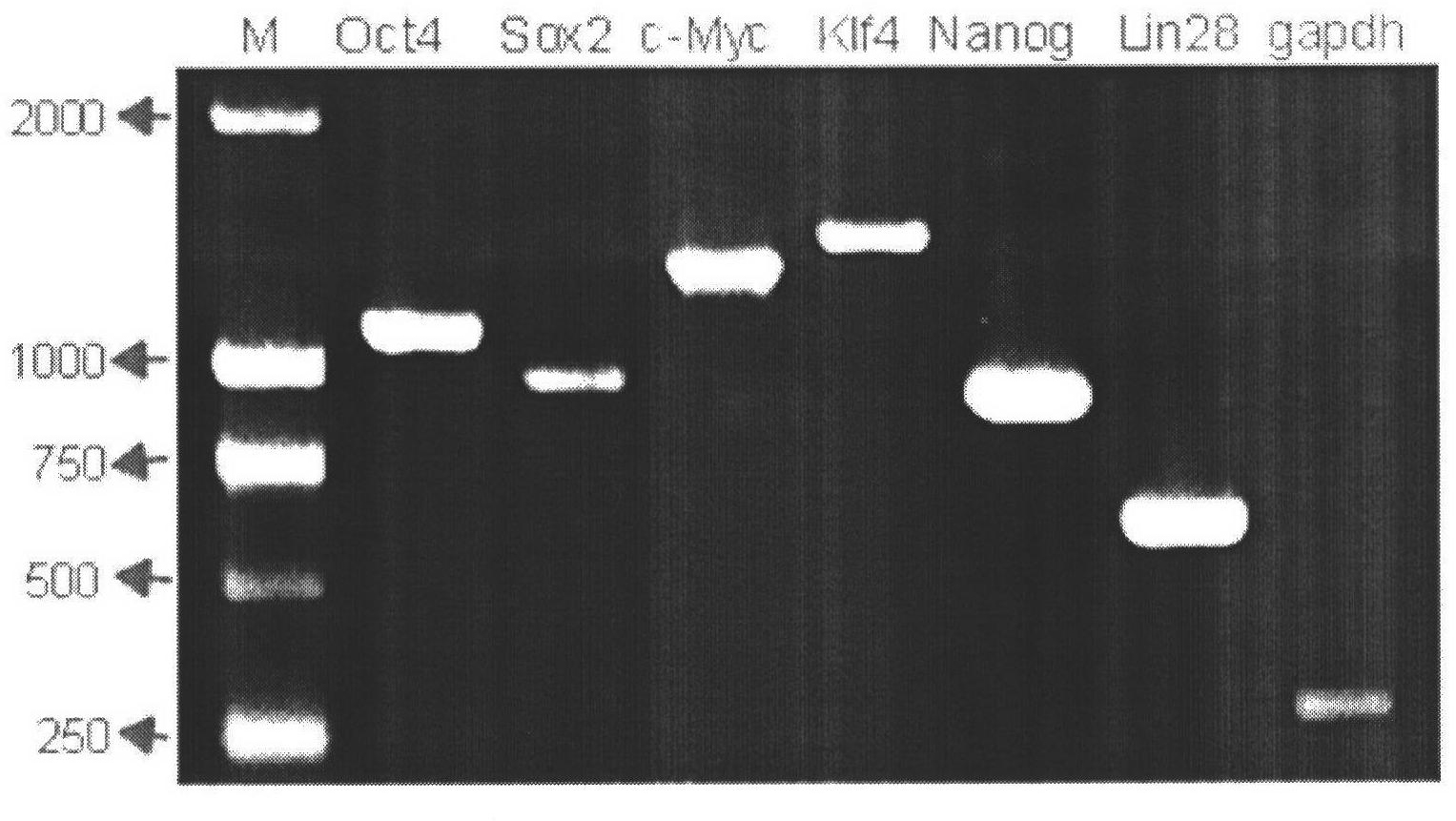
Production method for buffalo induced pluripotent stem cells
InactiveCN102433308AMake up for separationMake up for difficult cultivationFermentationGenetic engineeringReprogrammingLIN28
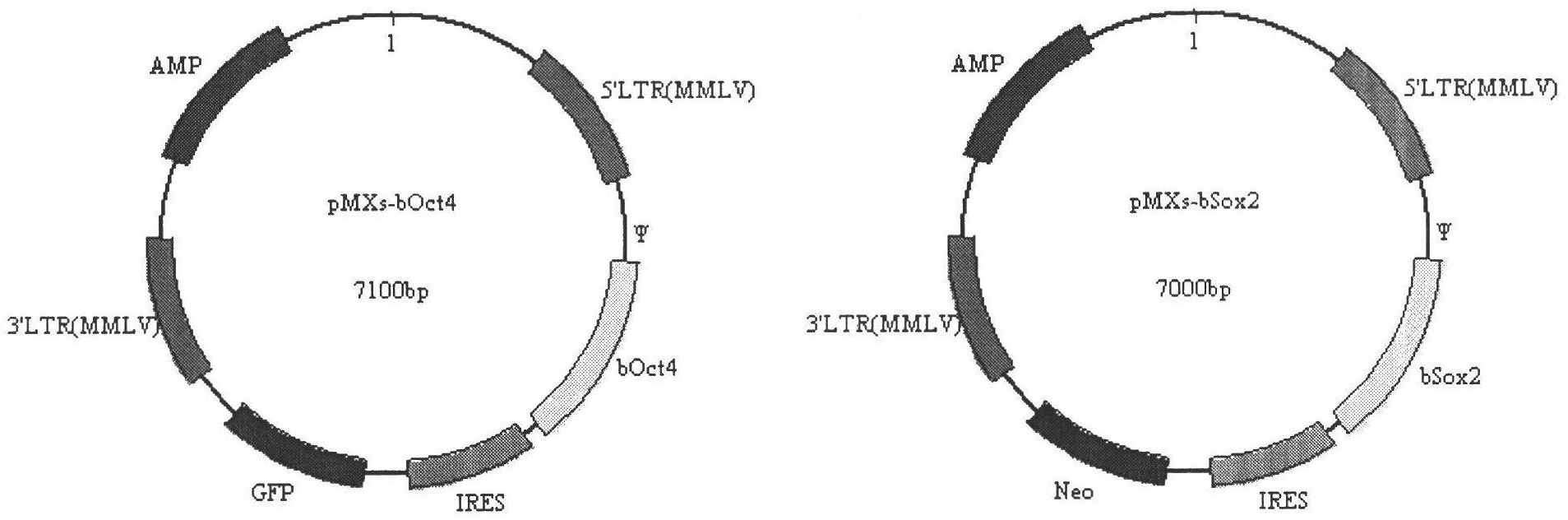
The invention discloses a production method for buffalo induced pluripotent stem cells. Specific transcription factors such as Oct4, Sox2, Klf4, c-Myc, Nanog and Lin28 and a cell immortal gene SV40large T (LT) are carried by retrovirus vectors, and buffalo somatic cells such as fetus fibroblasts are directly reprogrammed into the induced pluripotent stem cells with characteristics similar to those of embryonic stem cells. Due to the generation of the buffalo induced pluripotent stem cells, a new method for obtaining buffalo stem cells can be provided, the problems that the buffalo embryonic stem cells are difficult to separate and cultivate, and cell lines are difficult to establish are solved to some extent, moreover, sources of stem cells are provided for the research of signal paths of the buffalo stem cells and the establishment of the cell lines, and a new method is provided for directional genetic improvement of buffalos at the same time.
Owner:GUANGXI UNIV
Liver oval cell immortalization culture medium as well as preparation method and application thereof
PendingCN113583940AVigorous proliferative activityArtificial cell constructsVertebrate cellsCancer cellProliferation activity
The invention relates to the technical field of cancer cell culture, in particular to a liver oval cell immortalization culture medium and a preparation method and application thereof. The liver oval cell immortalization culture medium comprises basic components and additive factors, the additive factors comprise a hepatocyte growth factor, an epidermal growth factor and a leukemia inhibition factor. The liver cell growth factor, the epidermal growth factor and the leukemia inhibition factor are combined for use, so that the immortalization process of the mouse liver oval cells can be effectively promoted, the liver oval cells can at least maintain the cell proliferation activity for more than 8 weeks under the in-vitro condition, cell differentiation is avoided, and the cells are round or oval. According to the scheme, the culture medium can enable the hepatic oval cells to have the characteristic of repeated passage, can be applied to practical operation of maintaining the proliferative activity of the hepatic oval cells and inhibiting differentiation of the hepatic oval cells, and can provide more cell resources for researchers.
Owner:成都以邦医药科技有限公司
Lamb testicular sertoli cell immortalized cell line and its establishment method and application
ActiveCN109837250BExtend your lifeEfficient ProliferationMicroorganism based processesViruses/bacteriophagesCapripoxvirusTGE VACCINE
The invention discloses an immortalized cell line of lamb testis Sertoli cells and its establishment method and application. The lamb testis Sertoli cell immortalized cell line is named as lamb testis Sertoli cell immortalized cell line hTERT‑LSC, and its preservation number is CCTCC NO: C2018202. The experiment found that the hTERT-LSC cell line can be continuously passaged for more than 60 generations, and its growth and proliferation characteristics remain unchanged. The titer of sheeppox virus propagated by this cell line is equivalent to that of lamb testicular Sertoli cell LSC, and the cell division rate is higher than that of LSC, and the division rate can reach 1:3. At the same time, the immortalized cell line is used to prepare the vaccine, which simplifies the production process, shortens the production cycle, reduces the production cost, and ensures the stable quality of the prepared sheep pox virus vaccine. Therefore, the proposal of the present invention provides a new technical means for the large-scale production of the sheep pox virus vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Neonatal bovine testicular sertoli cell immortalized cell line and its establishment method and application
ActiveCN109735499BExtend your lifeThe characteristics of growth and proliferation are unchangedMicroorganism based processesViruses/bacteriophagesImpetigoTGE VACCINE
The invention discloses an immortalized cell line of neonatal bovine testis Sertoli cells, its establishment method and application. The immortalized Sertoli cell line of newborn bovine testis is named as the immortalized Sertoli cell line of newborn bovine testis hTERT‑NBSC, and its preservation number is CCTCC NO: C2018201. Experiments have found that hTERT-NBSC can be continuously passaged for more than 65 passages, and the growth and proliferation characteristics remain unchanged. The cell line is more sensitive to ORFV inoculation, can multiply ORFV virus efficiently, and can ensure the uniformity and stability of the virus. The virus titer of ORFV propagated by this cell line is equivalent to that of Sertoli cells of newborn bovine testis, and the cell division rate is higher than that of NBSC, and the division rate can reach 1:3. At the same time, the passage cell line is used to prepare the vaccine, which simplifies the production process, shortens the production cycle, reduces the production cost, and ensures the stable quality of the prepared sheep contagious impetigo vaccine. Therefore, the proposal of the present invention provides a new technical means for the large-scale production of the sheep contagious impetigo vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Fully humanized anti-hepatitis B virus monoclonal antibody as well as preparation method and application thereof
ActiveCN111620944ASimple screening methodDigestive systemImmunoglobulins against virusesHepatitis B virusGenetic engineering
The invention discloses a fully humanized anti-hepatitis B virus monoclonal antibody as well as a preparation method and an application thereof, and belongs to the technical field of biomedicine and genetic engineering antibody. The monoclonal antibody comprises a heavy chain variable region and a light chain variable region; the amino acid sequence of the heavy chain variable region is shown as SEQ ID NO. 2; the amino acid sequence of the light chain variable region is shown as SEQ ID NO: 4; the coding DNA of the antibody comprises a heavy chain variable region and a light chain variable region, wherein the coding DNA sequence of the heavy chain variable region is shown as SEQ ID NO. 1; and the coding DNA sequence of the light chain variable region is shown as SEQ ID NO: 3. According to the method, B cells are immortalized through EBV virus, the natural fully-humanized hepatitis B virus monoclonal antibody is screened by combining a limited dilution method, and the screening method does not need a large expensive instrument, is simple and easy to implement and is suitable for being carried out in a conventional biology laboratory.
Owner:CHANGCHUN NORMAL UNIVERSITY +1
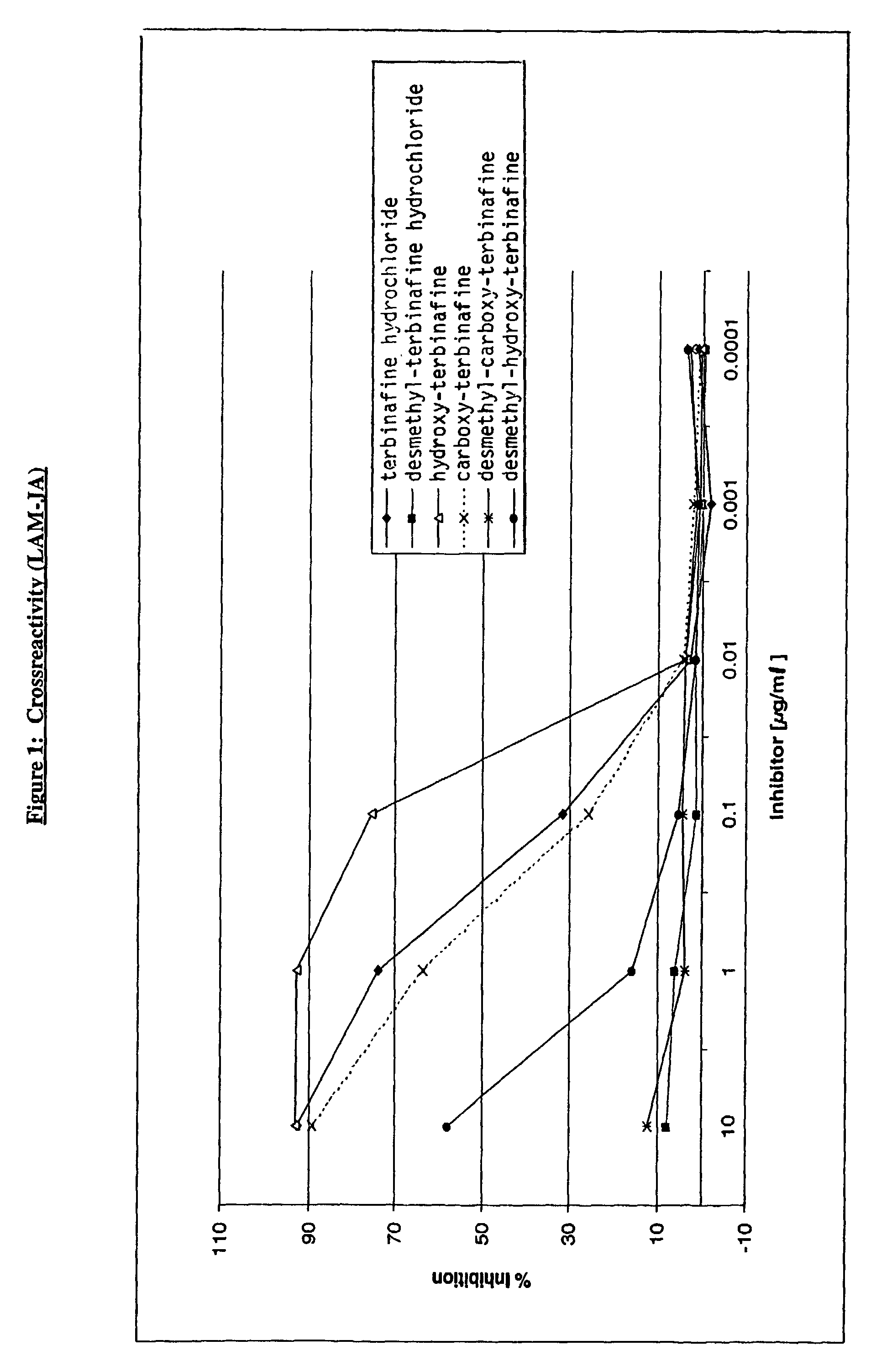
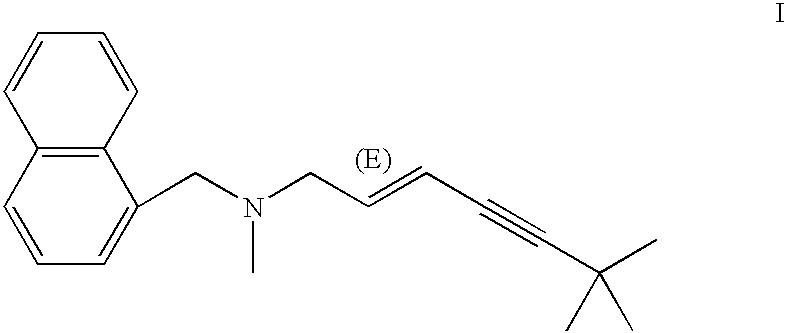
Specific monoclonal antibody against terbinafine
InactiveUS7122184B2Animal cellsImmunoglobulins against animals/humansTissue concentrationsMonoclonal antibody
The invention concerns momoclonal antibodies to terbinafine in free base or salt form, immunogenic conjugates suitable for preparing them, hybridoma capable of producing them, and corresponding assay kits. The antibodies can be prepared by administering to an appropriate animal an immunogenic conjugate of a suitable derivative of terbinafine covalently linked to an immonogen, recovering antibody-producing cells sensitized to the conjugate, immortalizing the antibody-producing cells, selecting a resultant immortalized cell line, and recovering the resultant antibodies therefrom. They are indicated for use in particular in the measurement of terbinafine tissue concentration and distribution in bodily fluids or compartments, especially nails.
Owner:NOVARTIS AG
Method for inducing differentiation of mesodermal stem cells, es cells, or immortalized mesodermal stem cells into neural cells
InactiveUS20090280564A1Improve treatment efficiencyHigh proliferation rateNervous disorderCulture processCell FractionNeural cell
Mesodermal stem cells or ES cells, prepared from the mononuclear cell fraction isolated from bone marrow fluid or umbilical blood, were found to differentiate into neural stem cells, neurons, or glial cells when cultured in a basal culture medium. In addition, the differentiation of the mesodermal stem cells or ES cells into neural cells was promoted through the addition of an ischemic brain extract to the above-mentioned basal culture medium. Furthermore, the neural cells obtained using the above-described method for inducing differentiation were revealed to have neural regeneration potency in a brain infarction model, a dementia model, a spinal cord injury model and a demyelination model.In addition, according to the present invention, mesodermal stem cells can be differentiated into neural cells by immortalizing the mesodermal stem cells by highly expressing or activating an immortalization gene in the mesodermal stem cells and culturing the cells under an appropriate condition. The methods of the present invention are very useful in the medical field of neural regeneration.
Owner:NC MEDICAL RES
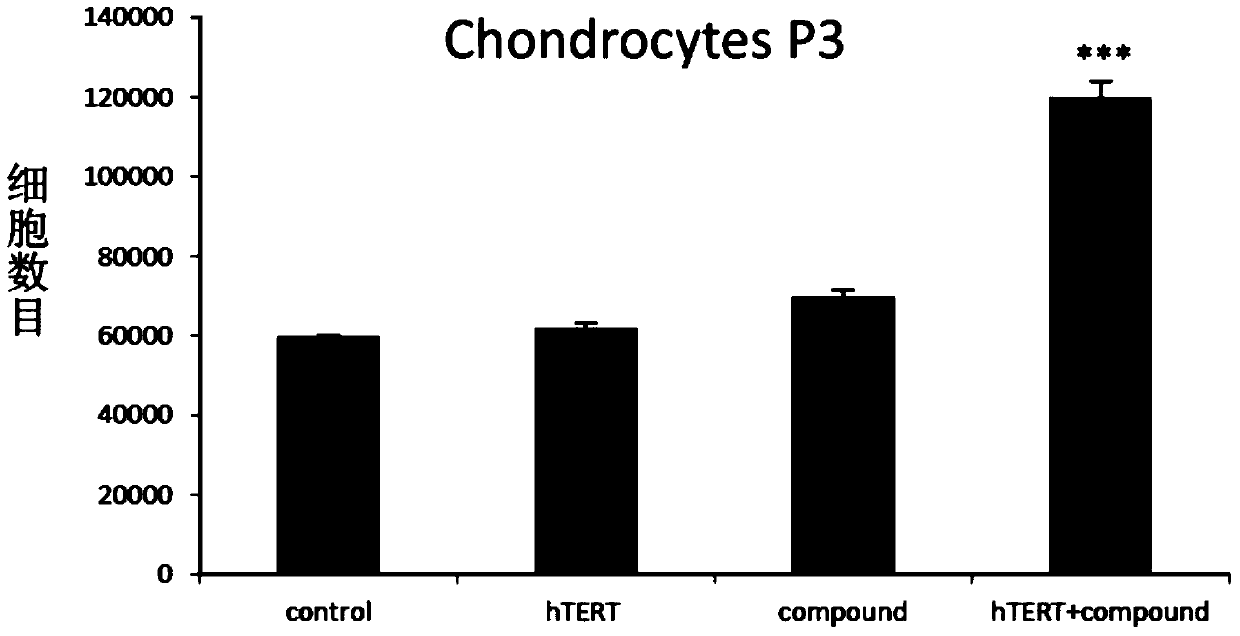
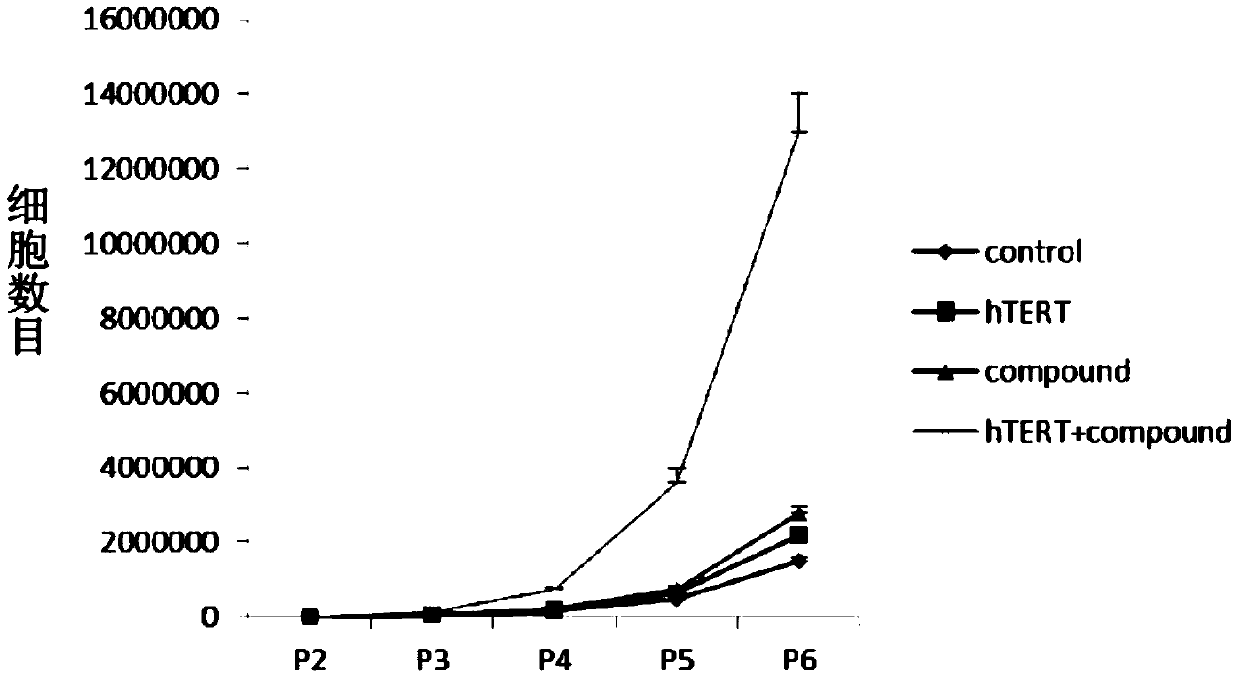
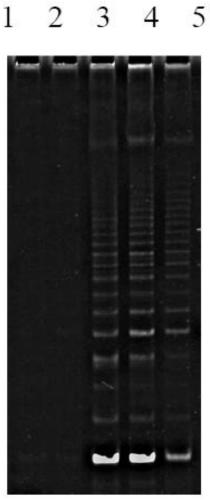
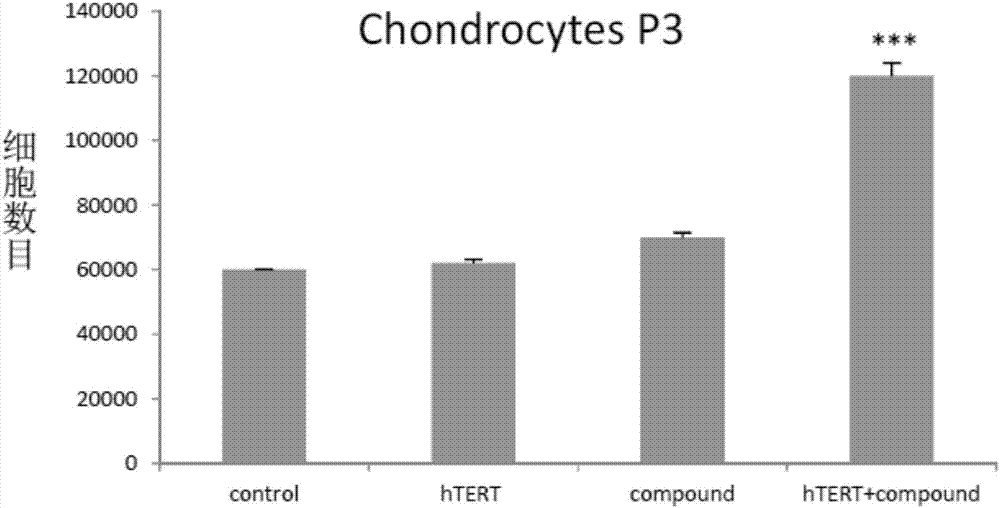
In vitro telomere extension and proliferation culture method of chondrocytes and human tissue engineered regeneration of cartilage
ActiveCN106967686BIncreased proliferationHigh activityGenetically modified cellsTransferasesCartilage cellsVitamin C
The invention discloses a method for lengthening, proliferating and culturing in-vitro telomeres of cartilage cells and human tissue engineering regenerated cartilage. The method and the human tissue engineering regenerated cartilage have the advantages that telomerase mRNA [(messenger RNA (ribonucleic acid)) transfection is carried out on the cartilage cells in an in-vitro manner, accordingly, the telomeres in cartilage cells obtained by means of culturing in follow-up procedures can be lengthened, proliferation and the viability of the cartilage cells and cartilage cells of elderly patients in particular can be obviously improved by the aid of synergistic effects of the telomerase mRNA transfection and small molecule compounds such as vitamin C, B18R or p65i, rapamycin and resveratrol in complete media, and the method and the human tissue engineering regenerated cartilage have important significance in improving or treating cell function decline due to senescence when the actually applied to regeneration medical treatment; the telomeres of the cartilage cells are not continuously or even frequently lengthened by the aid of the method for lengthening, proliferating and culturing the in-vitro telomeres of the cartilage cells, accordingly, genome insertion mutation risks or cell immortalization tumor formation risks can be prevented, the safety and the applicability can be greatly improved, and the method and the human tissue engineering regenerated cartilage can be effectively applied to the regeneration medical treatment.
Owner:BEIHAO STEM CELL & REGENERATIVE MEDICINE RES INST CO LTD +2
Method for lengthening, proliferating and culturing in-vitro telomeres of cartilage cells and human tissue engineering regenerated cartilage
ActiveCN106967686AIncreased proliferationHigh activityGenetically modified cellsCulture processCartilage cellsVitamin C
The invention discloses a method for lengthening, proliferating and culturing in-vitro telomeres of cartilage cells and human tissue engineering regenerated cartilage. The method and the human tissue engineering regenerated cartilage have the advantages that telomerase mRNA [(messenger RNA (ribonucleic acid)) transfection is carried out on the cartilage cells in an in-vitro manner, accordingly, the telomeres in cartilage cells obtained by means of culturing in follow-up procedures can be lengthened, proliferation and the viability of the cartilage cells and cartilage cells of elderly patients in particular can be obviously improved by the aid of synergistic effects of the telomerase mRNA transfection and small molecule compounds such as vitamin C, B18R or p65i, rapamycin and resveratrol in complete media, and the method and the human tissue engineering regenerated cartilage have important significance in improving or treating cell function decline due to senescence when the actually applied to regeneration medical treatment; the telomeres of the cartilage cells are not continuously or even frequently lengthened by the aid of the method for lengthening, proliferating and culturing the in-vitro telomeres of the cartilage cells, accordingly, genome insertion mutation risks or cell immortalization tumor formation risks can be prevented, the safety and the applicability can be greatly improved, and the method and the human tissue engineering regenerated cartilage can be effectively applied to the regeneration medical treatment.
Owner:BEIHAO STEM CELL & REGENERATIVE MEDICINE RES INST CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com