Diagnosis and treatment of cancer using Anti-itm2a antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
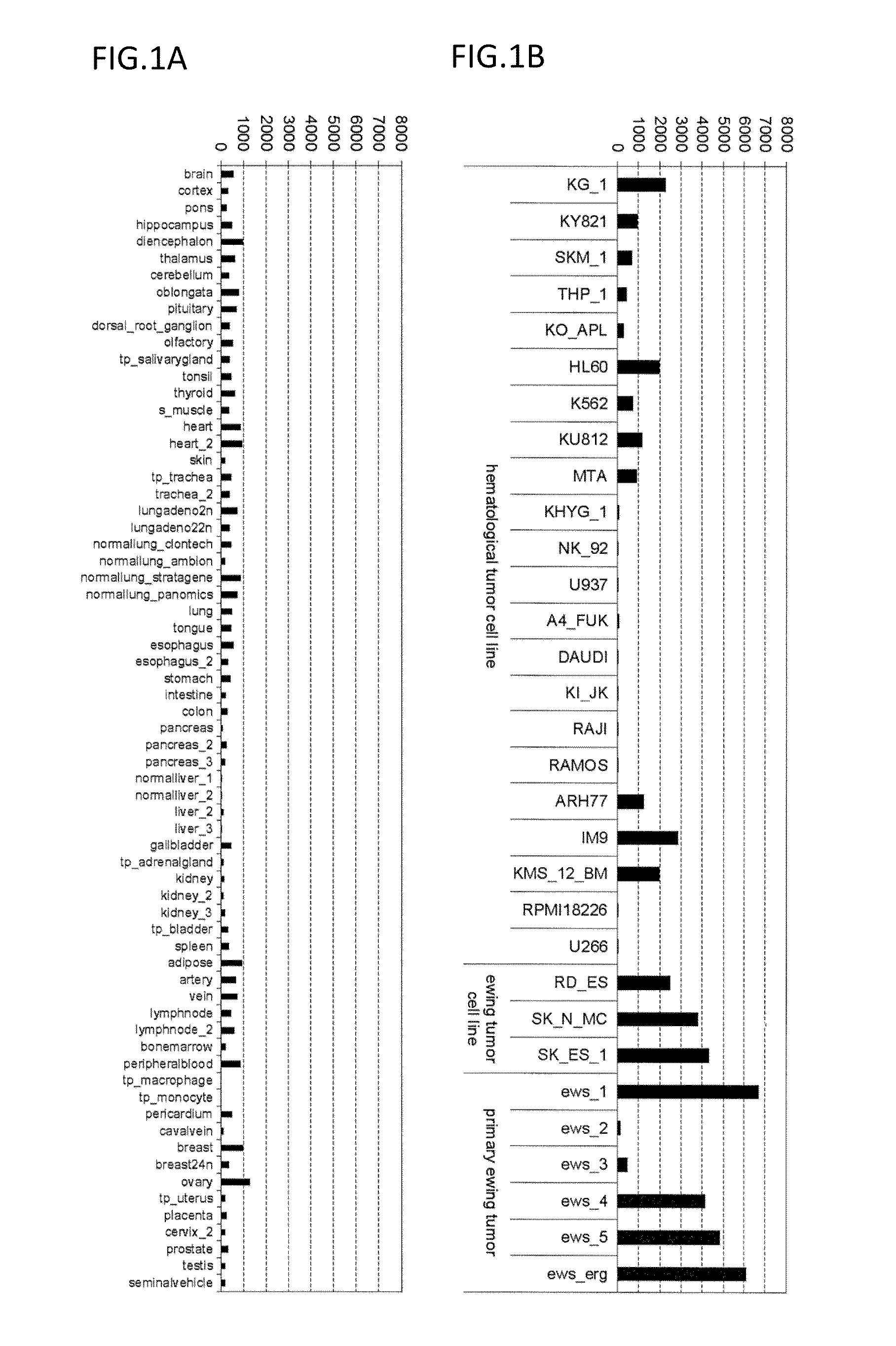
Expression Analysis of ITM2A mRNA
[0215]The expression of ITM2A mRNA was assayed in clinical Ewing's sarcoma samples, Ewing's sarcoma cell lines, blood cancer cell lines, and normal tissues using Human Exon 1.0 ST Array (Affymetrix, Inc.). The expression analysis employed 1 μg of total RNAs from each sample shown in FIG. 1. The analysis was conducted according to a method described in GeneChip Whole Transcript (WT) Sense Target Labeling Assay Manual (Affymetrix, Inc.). The data was digitized using Exon Array Computational Tool software (Affymetrix, Inc.). The total RNAs of normal tissues used in the analysis were normal tissues-derived total RNAs purchased from Clontech Laboratories, Inc., Ambion, Inc., Stratagene Corp., Cell Applications, Inc., Panomics, Inc., CHEMICON International, Inc., and BioChain Institute, Inc. Total RNAs were prepared from the tumor sites and normal sites of clinical cancer tissues (sampled after informed consent was obtained) and from cancer cell lines usin...
example 2
Preparation of Monoclonal Antibody Against ITM2A
[0217]cDNAs were prepared using SuperScript III Reverse Transcriptase (Invitrogen Corp.) with total RNAs prepared from a cancer cell line IM9 using Trizol as a template. A nucleotide sequence encoding ITM2A was amplified by PCR using the cDNAs as a template, a forward primer (SEQ ID NO: 43), and a reverse primer (SEQ ID NO: 44). This PCR employed PrimeSTAR GXL DNA Polymerase (Takara Bio Inc.) and was performed by 30 repetitive reaction cycles each involving 98° C. for 10 seconds, 55° C. for 15 seconds, and 68° C. for 1 minute. The amplification products formed from the PCR were cloned into pCR2.1-TOPO vectors (Invitrogen Corp.) to obtain pCR2.1_ITM2A. The inserted sequence of pCR2.1_ITM2A was sequenced to confirm that the inserted sequence was the same as a sequence registered under RefSeq Accession No. NM—004867.4.
(2-2) Preparation of Expression Vector for DNA Immunization
[0218]A nucleotide sequence encoding...
example 3
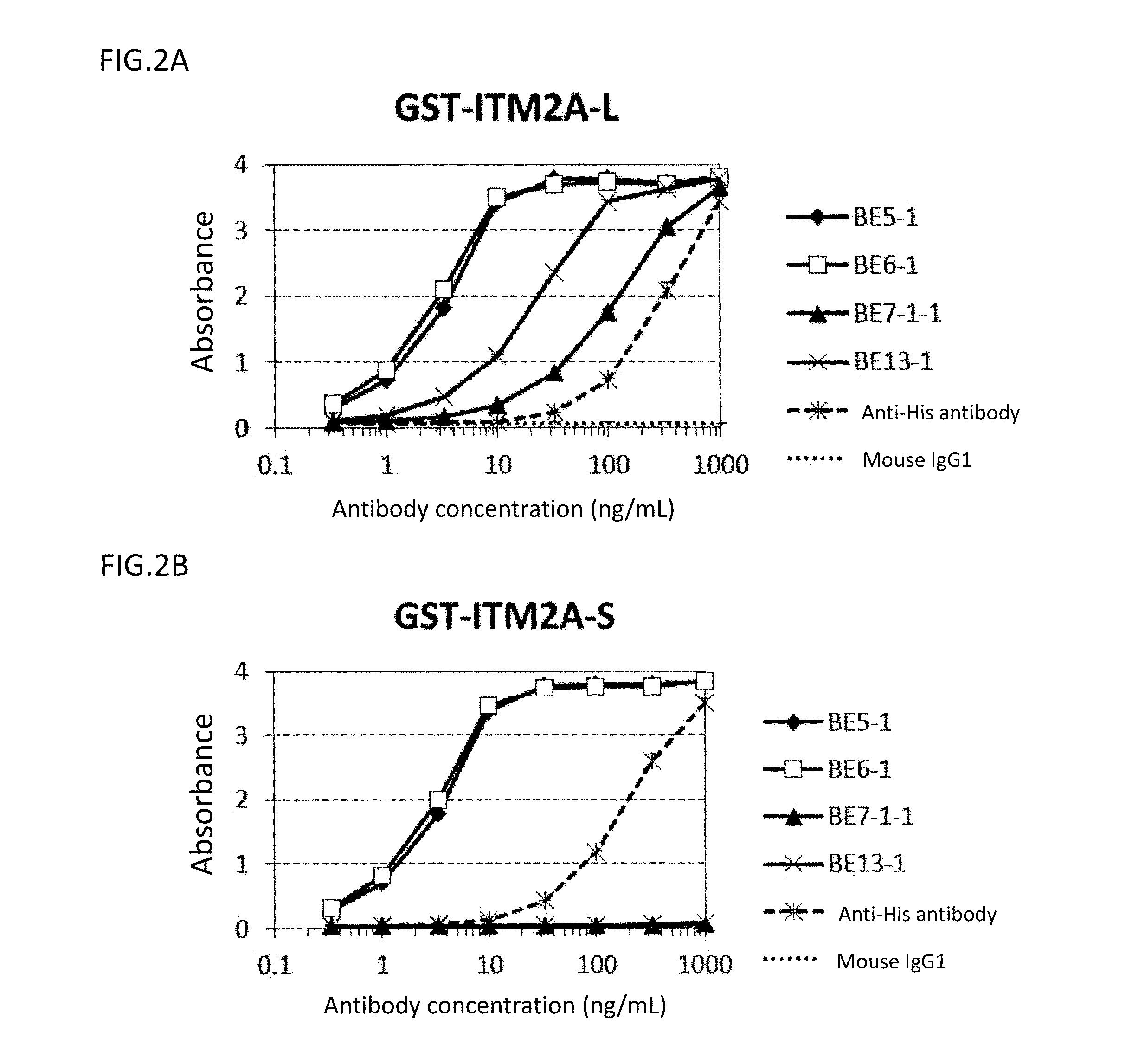
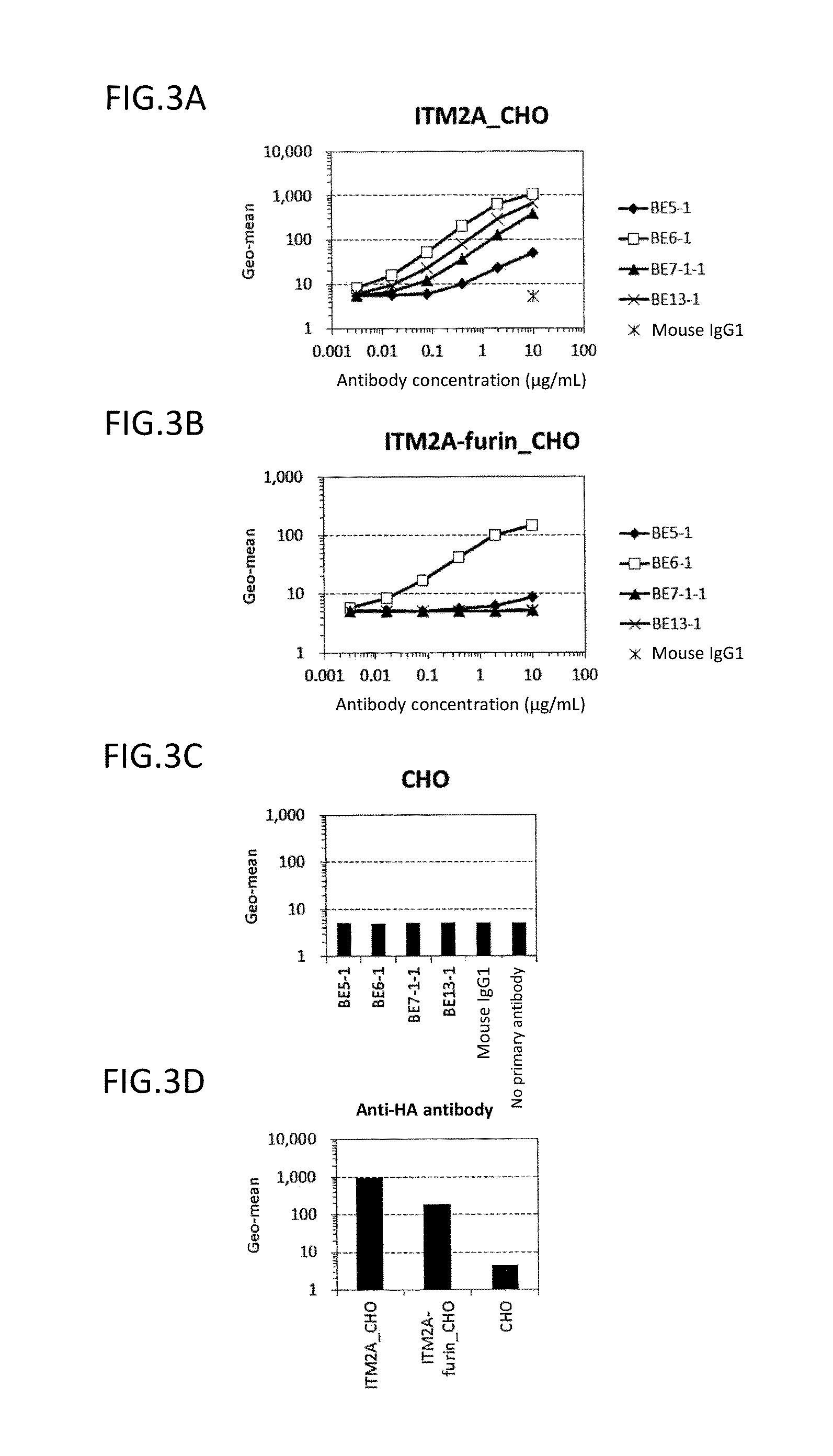
Analysis on Epitope for Anti-ITM2A Monoclonal Antibody by ELISA
(3-1) Preparation of Partial ITM2A Protein
[0224]The ITM2A extracellular region (Tyr75-Glu263) or a portion thereof (Tyr75-Lys182) was expressed as a GST-fusion protein in E. coli (Tyr75-Glu263: GST-ITM2A-L, and Tyr75-Lys182: GST-ITM2A-S). The fusion protein was C-terminally His-tagged. First, a nucleotide sequence encoding ITM2A (Tyr75-Glu263) or ITM2A (Tyr75-Lys182) was amplified by PCR using pCR2.1_ITM2A as a template, a forward primer (SEQ ID NO: 57) having EcoRI site, and a reverse primer (SEQ ID NO: 58 or 59) having SalI site and a His tag sequence, and cloned into pCR2.1-TOPO vectors. EcoRI / SalI-digested fragments of the plasmids obtained as a result of the cloning were cloned into the EcoRI-SalI sites of pGEX6P-1 vectors (GE Healthcare Bio-Sciences Corp.) to obtain plasmids pGEX_GST-ITM2A-L and pGEX_GST-ITM2A-S, respectively. The nucleotide sequence from start codon to stop codon in pGEX_GST-ITM2A-L is shown in SE...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com