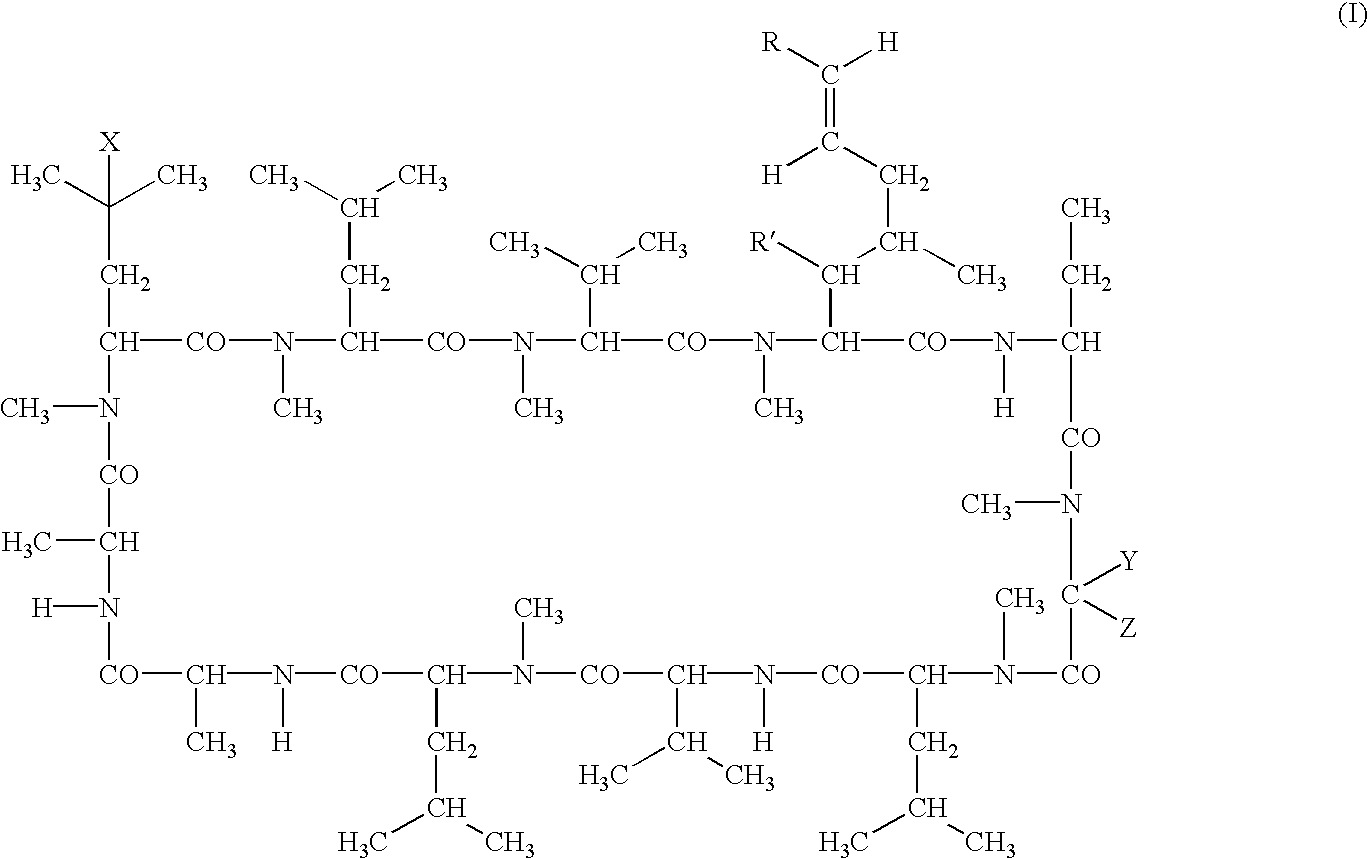
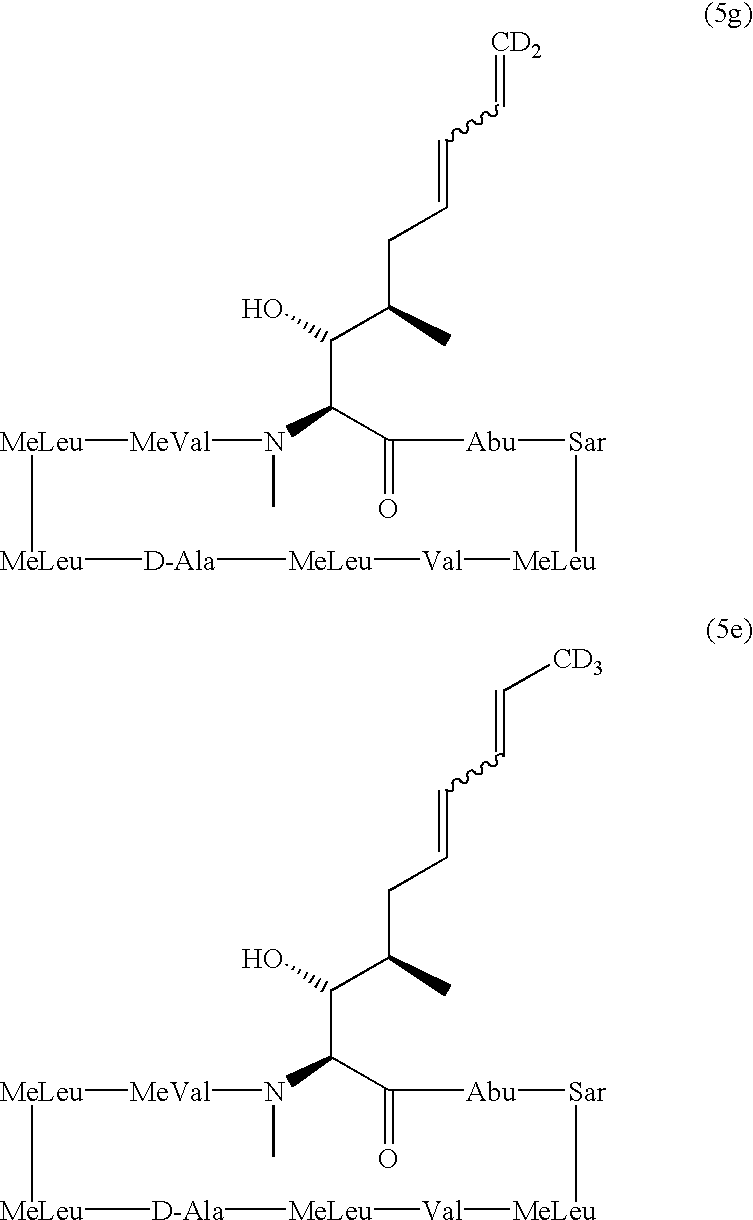
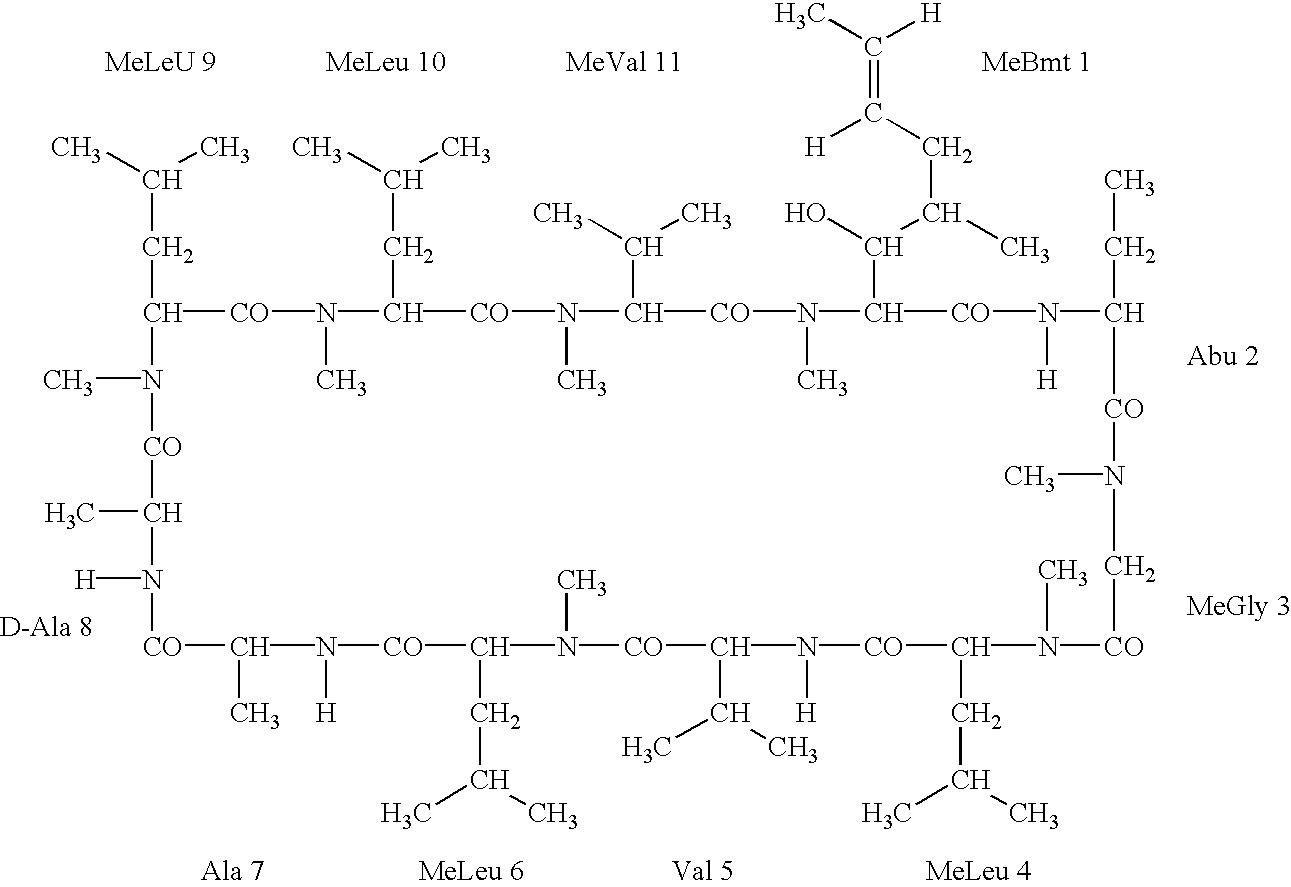Deuterated cyclosporine analogs and their use as immunomodulating agents
a cyclosporine analog and immunomodulating agent technology, applied in the field of cyclosporin derivatives, can solve the problem of not doing anything to modify the immunologic basis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0073] To a stirred solution of cyclosporine 1(1.01 g, 0.84 mmol) in acetic anhydride (20 mL) at room temperature was added DMAP (150 mg, 1.23 mmol 1.5 eq). After stirring overnight, the reaction mixture was partitioned between EtOAc (50 ml) and water (25 ml). The separated EtOAc layer was then washed with water (50 mL) and brine (50 mL), dried (MgSO.sub.4) and the solvent removed in vacuo to give the crude product as a glassy solid. Purification by flash chromatography through a short column of silica (2% MeOH / DCM) and lyophilisation from benzene yielded 2 (1.044 g, 0.84 mmol, quant.) as a fluffy, colourless solid; [.alpha.].sub.D.sup.25-305.7 (c. 0.3, CHCl.sub.3); .nu..sub.max (CHCl.sub.3 cast) / cm.sup.-1 3328m, 2963m, 1746m, 1627s, 1528m, 1472m, 1233m; .delta..sub.H (600 MHz, C.sub.6D.sub.6) 8 73 (1H, d, J=9.5 Hz, NH, 8.30 (1H, d, J=7.0 Hz, NH), 7.92 (1H, d, J=7.5 Hz, NH), 7.49 (1H, d, J=7.5 Hz, NH), 6.05 (1H, d, J=11.5 Hz), 5.88 (1H, dd, J=3.5, 11.5 Hz), 5.82 (1H, d, J=11.5 Hz), ...
example 2
[0074] To a solution of compound 2 (289 mg, 0.23 mmol) in a 1:1 mixture of dioxane and water (5 mL) was added firstly sodium metaperiodate (100 mg, 0.47 mmol, 2 eq) and secondly a solution of osmium tetraoxide (5 mL; 0.5 g OsO.sub.4 in 250 mL of solvent). Two-phase work-up, purification by flash column chromatography (40% acetone in petroleum ether) and lyophilisation from benzene gave compound 3. (226 mg, 0.18 mmol, 80%) as a fluffy, colourless solid; [.alpha.].sub.D.sup.25-260.0 (c. 0.1, CHCl.sub.3); .nu..sub.max (CHCl.sub.3 cast) / cm.sup.-1 3325m, 2962m, 1748w, 1724w, 1677m, 1626s, 1228m, 755m; .delta..sub.H (300 MHz, C.sub.6D.sub.6) 8.63 (1H, d, J=9.5 Hz, NH), 8.16 (1H, d, J=7.0 Hz, NH), 7.95 (1H, d, J=7.5 Hz, NH) 7.48 (1H, d, J=9.0 Hz, NH), 5.93 (1H, d, J=7.5 Hz), 5.84 (1H, dd, J=4.0, 11.5 Hz), 5.70 (1H, d, J=11.5 Hz). 5.56-5.54 (1H, m), 5.32 (1H, dd, J=5.5, 8.0 Hz), 5.07-4.88(3H, complex), 4.72 (1H, p, J=7.0 Hz), 4.49 (1H, p, J=7.0 Hz), 3.98 (1H, d, J=14.0 Hz), 3.42 (3H, s, CH....
example 3
[0075] Method A: To a solution of compound 3(315 mg, 0.26 mmol) in THF (5 mL) at 0.degree. C. was added a solution of the deutero-phosphorus ylid (2.67 mmol, .about.10 eq), prepared from d.sub.5-ethyltriphenylphosphoniu-m iodide. After work-up, purification by flash column chromatography (30% to 60% acetone in PE) and HPLC (60% to 65% MeCN in water), then lyophilisation from benzene yielded compound 4 (153 mg, 0.12 mmol, 47%) as a fluffy, colourless solid.
[0076] Method B: To a stirred solution of compound 3 (287 mg, 0.23 mmol) in THF (5 mL) under Ar at -78.degree. C. was carefully added a solution of phosphorus ylid (formed by the addition of sodium hexamethyldisilylamide (1.0M; 2.25 mL, 2.25 mmol, .about.10 eq) to a suspension of d.sub.5-ethyltriphenylphosphonium iodide (480 mg, 1.13 mmol, .about.5 eq) in THF (10 mL) under Ar at room temperature). After stirring for 2 hr with gradual warming to room temperature, the reaction mixture was cooled to 0.degree. C. and was quenched by th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com