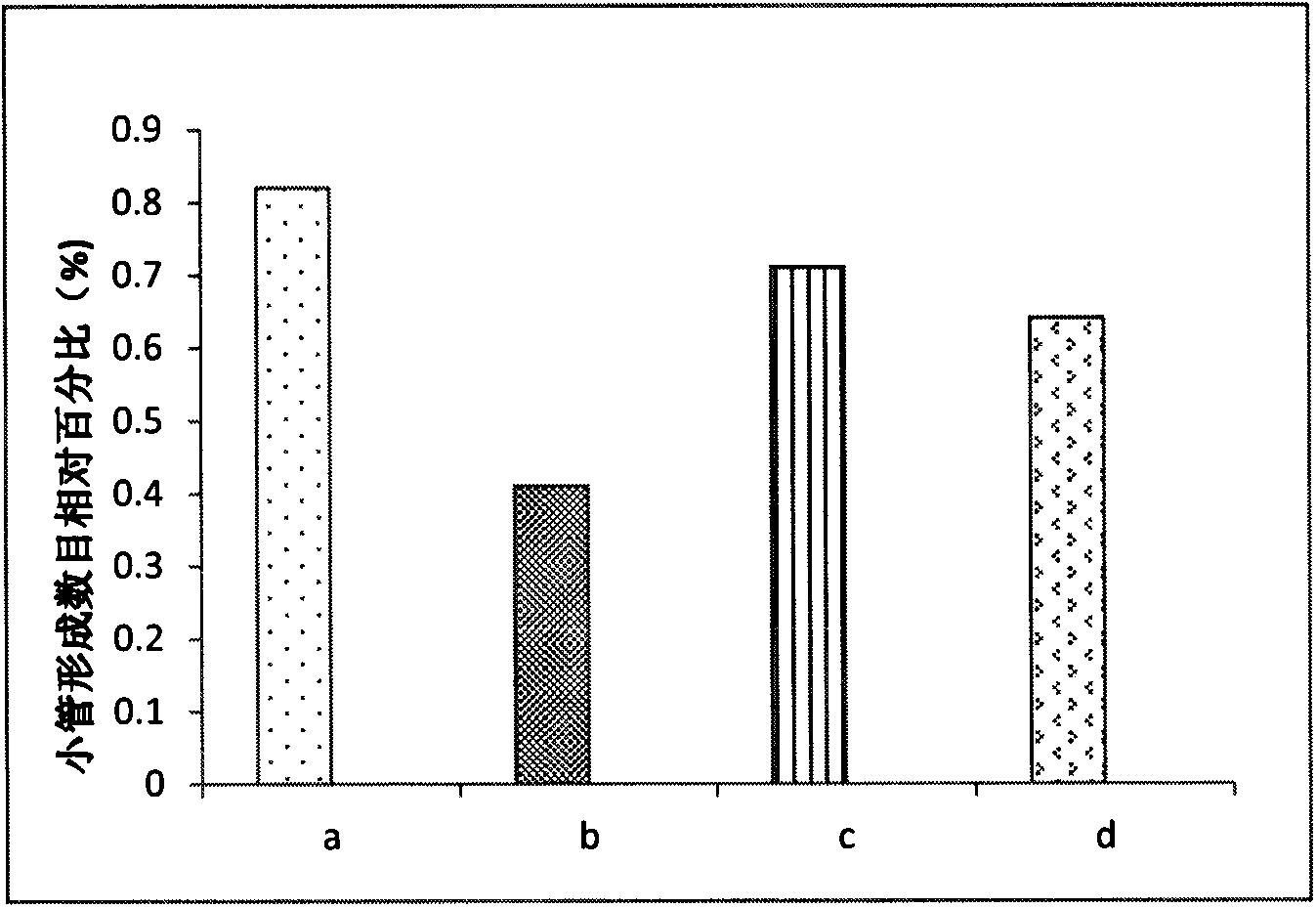
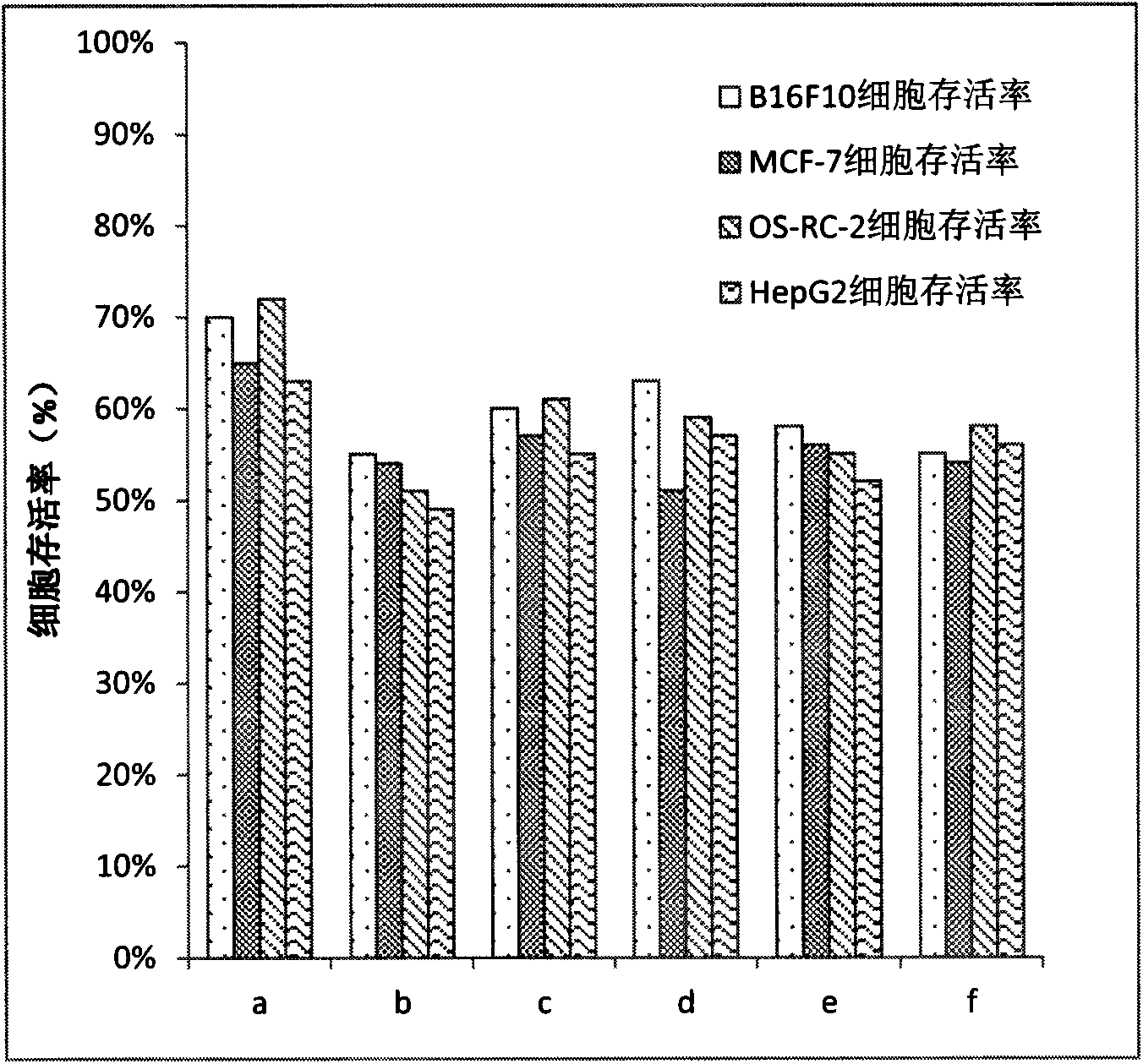
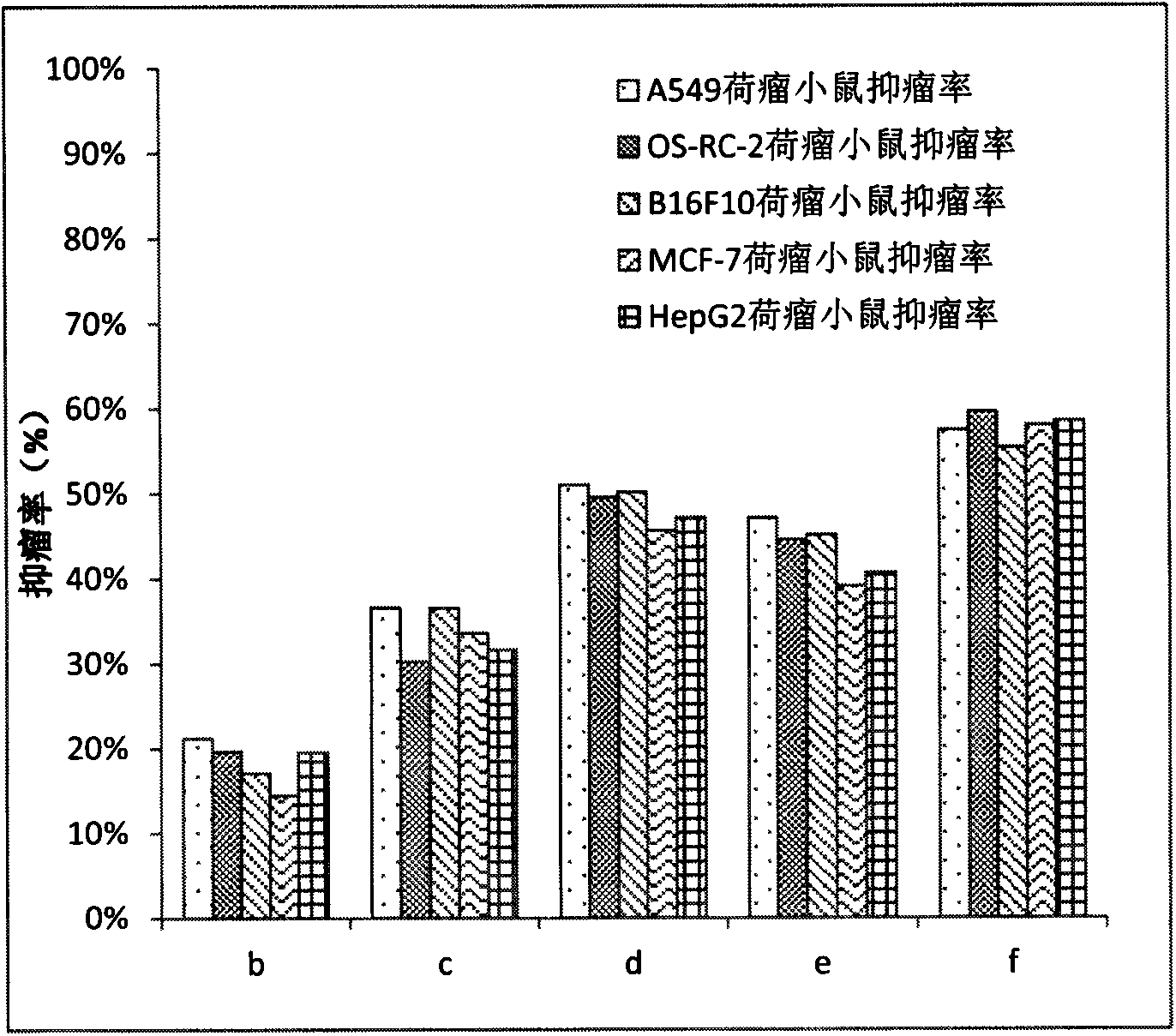
Preparation of amphiphilic ursolic acid-polysaccharide coupled substance and application thereof in treating tumors
A kind of ursolic acid and amphiphilic technology, which is applied in the field of preparation and its application in tumor treatment, can solve the problems of difficult preparation of preparations and low bioavailability, and achieve improved water solubility and stability, high drug loading, and The effect of prolonging time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 The synthesis of amphiphilic ursolic acid-low molecular weight heparin conjugate
[0058] 300mg of ursolic acid was dissolved in dichloromethane, and gradually dropped into the dichloromethane solution in which 280mg of pyridinium chlorochromate was dissolved at 10°C. After the dropwise addition was completed, stir at room temperature for 1.5h. The reaction solution was filtered, washed, washed with water, dried, filtered and concentrated, followed by silica gel column chromatography, eluted and concentrated to obtain a white solid. Take 59.2mg of the white solid and 80mg of hydroxylamine hydrochloride and dissolve it in 7ml of pyridine, connect it to a drying tube, and reflux at 115°C for about 6h. After the reaction solution was cooled to room temperature, it was washed with distilled water, filtered, washed with water, and dried to obtain a white solid. Take 120 mg of the white solid, 1.03 g of sodium cyanoborohydride, and 1.03 g of sodium acetate, and...
Embodiment 2
[0059] Example 2 Synthesis of amphiphilic ursolic acid-desulfated heparin conjugate
[0060] 250mg of ursolic acid was dissolved in dichloromethane, and gradually dropped into the dichloromethane solution in which 270mg of pyridinium chlorochromate was dissolved at 12°C. After the dropwise addition was completed, stir at room temperature for 5 h. The reaction solution was filtered, washed, washed with water, dried, filtered and concentrated, followed by silica gel column chromatography, eluted and concentrated to obtain a white solid. Take 61.3mg of the white solid and 81mg of hydroxylamine hydrochloride and dissolve it in 8ml of pyridine, connect it to a drying tube, and reflux at 110°C for about 7h. After the reaction solution was cooled to room temperature, it was washed with distilled water, filtered, washed with water, and dried to obtain a white solid. Take 150 mg of the white solid, 1.2 g of sodium cyanoborohydride, and 1.2 g of sodium acetate, and dissolve it in meth...
Embodiment 3
[0061] Example 3 Synthesis of amphiphilic ursolic acid-unfractionated heparin conjugate
[0062] 250mg of ursolic acid was dissolved in dichloromethane, and gradually dropped into the dichloromethane solution in which 270mg of pyridinium chlorochromate was dissolved at 5°C. After the dropwise addition, stir at room temperature for 4h. The reaction solution was filtered, washed, washed with water, dried, filtered and concentrated, followed by silica gel column chromatography, eluted and concentrated to obtain a white solid. Take 61.3mg of the white solid and 81mg of hydroxylamine hydrochloride and dissolve it in 8ml of pyridine, connect it to a drying tube, and reflux at 90°C for about 13h. After the reaction solution was cooled to room temperature, it was washed with distilled water, filtered, washed with water, and dried to obtain a white solid. Take 150 mg of the white solid, 1.2 g of sodium cyanoborohydride, and 1.2 g of sodium acetate, and dissolve it in methanol. The re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com