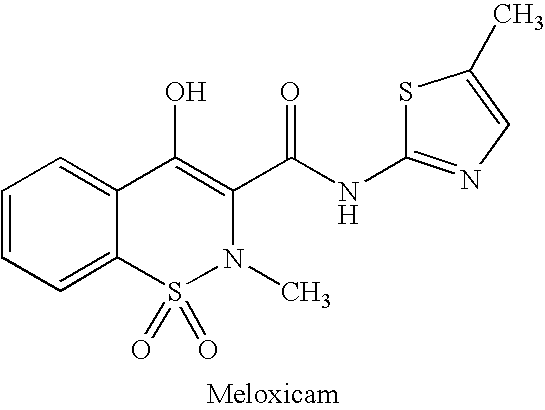
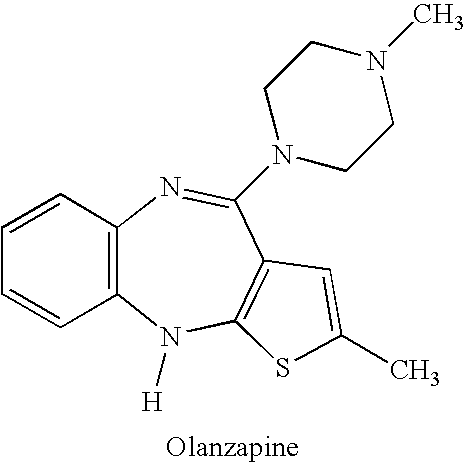
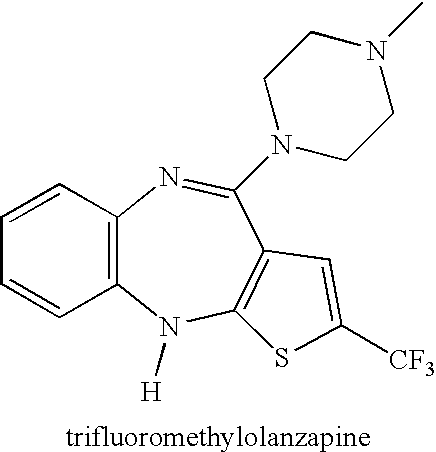
Advanced drug development and manufacturing
a technology for drug development and manufacturing, applied in the direction of material analysis using wave/particle radiation, instruments, x/gamma/cosmic radiation measurement, etc., can solve the problem of not being able to predict which chemicals will bind effectively to proteins, the same binding affinity, and the inability to validate the chemical
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first example
[0073]We measured the interaction between biotin and avidin to demonstrate that RPM could quantify the sulfur-containing small molecule (biotin, C10H16N2O3S) over the sulfur content of a protein (tetrameric avidin, Sigma-Aldrich, 16 sulfur atoms, see Korpela J. Avidin, a high affinity biotin-binding protein, as a tool and subject of biological research. Medical Biol. 1984, 62:5-26). Previous studies have shown that the ratio of sulfur in avidin-biotin:avidin is 19.5:16, or 1.22, see Green N M. Purification of Avidin. Meth Enzymol. 1970, XVIII(A):414-417. We incubated 500 micrograms of avidin in 1.0 ml of 0.20 millimolar biotin at pH 8.0 for 5 minutes and then centrifuged the sample for 3 hours at 7000 g using a Centricon YM-3 resulting in a concentrated avidin-biotin sample of 25-50 μl. We diluted the sample to 0.75 ml in TRIS buffer (pH 8.0), centrifuged for 2 hours at 7000 g, and repeated this process twice. We treated an avidin control sample identically. We used RPM to measure t...
second example
[0074]Our preliminary data show that RPM measures non-covalent protein-inhibitor complexes. We used di-zinc aminopeptidase (aAP) and its competitive inhibitor leucine phosphonic acid (LPA). LPA binds to aAP with a Ki of 6.6 μM at pH 8.0, see Stamper C et al. Inhibition of the aminopeptidase from Aeromonas proteolytica by L-leucinephosphonic acid. Spectroscopic and crystallographic characterization of the transition state of peptide hydrolysis. Biochemistry, 2001, 40:7035-7046. We incubated 500 micrograms of aAP in 1.0 mls of 0.20 millimolar LPA (>10×Ki) at pH 8.0 for 5 minutes. We centrifuged the aAP-LPA sample for 3 hours at 7000 g using a Centricon YM-3 resulting in a concentrated sample of 25-50 μl. We diluted this sample to 0.75 ml in TRIS buffer (pH 8.0), centrifuged for 2 hours at 7000 g, and repeated this process twice. We subjected two negative control samples to the same filtering conditions described above: The first control was 1.0 ml of 0.20 millimolar LPA, and the secon...
example 1
[0176]Binding of ziprasidone to protein receptors. A protein array may be prepared as described in G. MacBeath and S. L. Schreiber, “Printing Proteins As Microarrays For High-Throughput Function Determination,”Science, vol. 289, pp. 1760-1763, (2000). This protein array may be exposed to a solution of ziprasidone, which is the active ingredient in the drug Geodon™. Ziprasidone has a molecular formula of C21H20ClN4OS and the structure shown below.
[0177]The elements chlorine and sulfur may each be detected by x-ray fluorescence. After the array is exposed to the ziprasidone-containing solution, an EDAX Eagle Microprobe x-ray fluorescence microscope, for example, could be used to determine the receptor proteins that bind to the ziprasidone. The amount of ziprasidone that is associated with each receptor may be quantified by x-ray fluorescence.
[0178]None of the proteins of the array are expected to contain the element chlorine, so the chlorine x-ray fluorescence baseline may be assumed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com