Method for preparing genotype F mumps attenuated live vaccine
An attenuated live vaccine, mumps technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, inactivation/attenuation, etc., can solve problems such as allergic reactions, affecting product stability, and differences in virus sensitivity, and achieve The effect of good security
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1F genotype mumps live attenuated vaccine and its preparation method
[0026] Take the human embryonic lung diploid cells MHL-3 (MHL-3 purchased from Beijing Minhai Biotechnology Co., Ltd.) that grows into a single layer, and digest them with 0.25% trypsin solution. After the cells are soaked in trypsin solution, they form single cells. , Pour out the trypsin solution in the cell flask, add MEM cell culture solution pre-warmed at 37℃ and dilute to 10 5 Cells / mL. Then inoculate it in the cell factory and place it at 37°C for 24-48h.
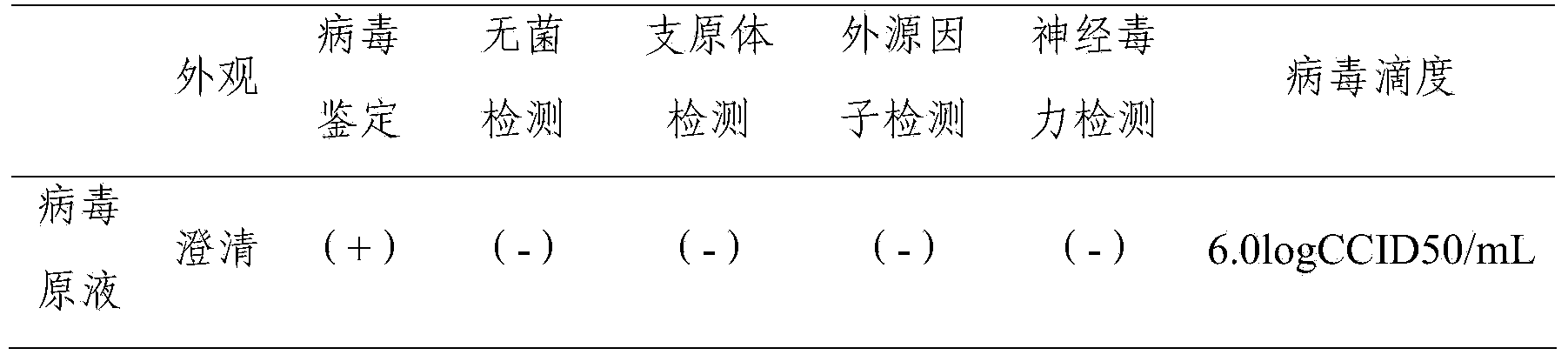
[0027] When the MHL-3 cells grow into a monolayer, discard the original culture medium, and wash the cell surface and the side wall of the cell factory with Earl's solution. F-genotype mumps virus MHM-19 suspension was inoculated at 0.1 MOI, and MEM maintenance solution was added to culture at 33°C.
[0028] Among them, the MEM culture medium contains 0.03% glutamine, 0.08% sodium bicarbonate, and 10% calf serum; the MEM maintenance medi...
Embodiment 2
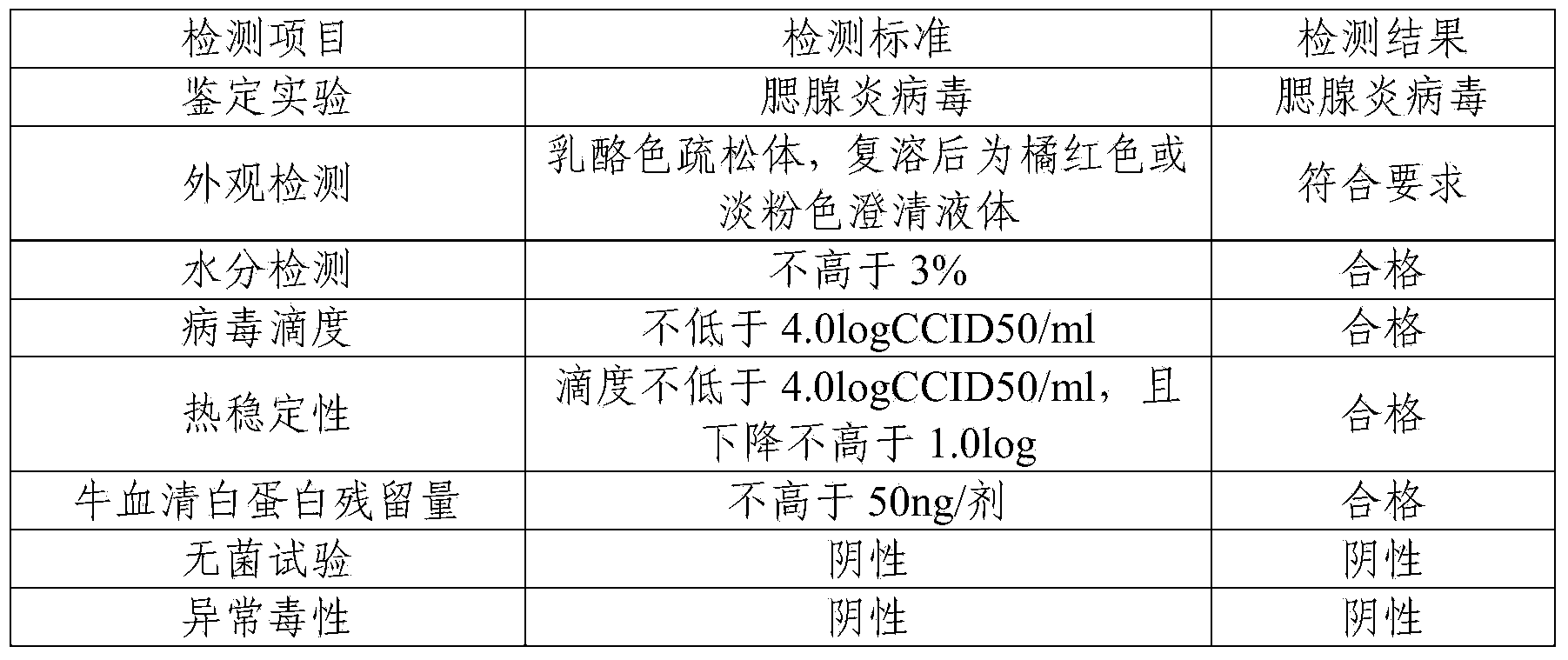
[0037] Example 2 Quality Inspection of F Genotype Mumps Live Attenuated Vaccine
[0038] The finished product of F genotype mumps live attenuated vaccine prepared in Example 1 was tested. The test items include: identification test, appearance inspection, moisture test, virus titer, heat stability test, bovine serum albumin residue test, sterility test, and abnormal toxicity test. The test results are shown in Table 2.
[0039] Table 2 F genotype mumps live attenuated live vaccine related test results
[0040]
Embodiment 3
[0041] Example 3 Immunogenicity analysis of F genotype mumps live attenuated vaccine
[0042] Fifteen rhesus monkeys (age 2-3 years old, weight 3kg±0.5kg) negative for mumps antibody were screened, and then divided into 3 groups, MHM-19 strain (prepared in Example 1) and S79 mumps attenuated live The vaccines were used to immunize a group of rhesus monkeys, and the negative control group was injected with 0.5ml DMEM. The immunization method was subcutaneous. At 2, 4, 8, 12, and 24 weeks after immunization, blood was collected to separate serum for neutralizing antibody determination. The results are shown in Table 3. The serum neutralizing antibody titers of the negative control group were all less than 2, which was negative. Both the MHM-19 and S79 experimental groups had good neutralizing antibody responses, and the immune effects of MHM-19 and S79 strains Significantly different (P <0.05). During the test period, the rhesus monkeys in each test group had no signs of parotid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com